Introduction
Among the properties of cyanobacteria (CB), which can be used in biotechnology aspect, refers to their ability to synthesize biologically active substances released in environment in the form of exometabolites. CB allocate its metabolites not only after the death of the cells, but also in the process of their normal physiological activity. The allocation of metabolites by the CB is one of the adaptations to changes in external conditions for them (Сакевич, 1985). The first detailed reviews of the chemical nature of such substances in the Russian literature are summarized in monographs (Сиренко, Козицкая, 1988; Андреюк и др., 1990). However, in these reviews, as in more later studies, was mainly provide information about the structure and properties, including toxinogenic aggressiveness, substances formed mainly by aqueous forms of CB (Румянцев, Крюков, 2012; Белых и др., 2015; Voloshko et al., 2008; Wejnerowski et al., 2018; Rott et al., 2018). The data about exogenous metabolites of the soil CB is not so much. At the same time the soil CB for many years are used as agents of becoming suppressiveness of chemically and biologically polluted soils, as well as for inoculation of seeds of various agricultural, forest and ornamental cultures (Домрачева, 2005; Домрачева и др., 2009). The results obtained about the positive impact of the introduction of the CB on increase of productivity of plants, uppression of the activity of phytopathogens, their remediation activity in soils contaminated with heavy metal ions, pesticides, petroleum products, require further in-depth study of the chemical composition of CB exometabolites. In particular, high metabolic activity and ecological potential is noted in the CB of the genus Nostoc (Огородникова и др., 2010; Фокина и др., 2015; Mezanka, Dembitsky, 2006).
The aim of this work is to reveal the composition and influence of exometabolites of soil cyanobacteria Nostoc paludosum Kütz 18 on the growth and development of the barley of sort Emerald in the presence of copper sulfate (II) and without it.
Materials
In this work were used:
– two-month-old CB culture of N. paludosum 18 with a titer of 3·107 cells/cm3, that was grown on Gromov’s liquid medium No. 6 without nitrogen;
– the cultural liquid (CL) N. paludosum 18;
– spring barley of sort Emerald. It was bred in the treatment of barley seeds of sort BIOS – 1 by the biological preparation "Agate 25 K".
For the experiment the CB culture was homogenized for 2 minutes using a homogenizer HG-15A-Set-A (DAIHAN Scientific, South Korea) at 30 thousand rpm. Then, a suspension of CB was used, its CL (separated from microorganisms by centrifugation) and extracts from CL, prepared by extraction of substances from CL to hexane (C6H14) and carbon tetrachloride (CTC, CCl4). Extraction by hexane is one of the most common ways to extract biologically active substances (carotene, carotenoids, tocopherols, flavonoids, etc.) from plant samples by chemically indifferent neutral organic solvent (Ивкова, Петрова, 2012). Carbon tetrachloride is a proven non-selective extractant, that is capable to dissolve many not only monomers, but also the compounds-polymers (Химическая энциклопедия..., 2012). The extraction was carried out in a ratio of CL: extractant, equal to 1:1, by portions with their following connection.
Determination of the composition of organic substances in CL, extracts from it and plant tissues. The determination of the composition of organic substances in the studied substrates was carried out by the method of high-performance liquid chromatography on the chromatograph Shimadzu LC-20, Prominence series with diode-matrix detector and gas chromatography on GC-2014 Shimadzu chromatograph with detector TCD-2014 (Japan).
Methods
Investigation of the influence of exometabolites of soil cyanobacteria N. paludosum on the growth and development of barley plantules
The filter paper was impregnated with the studied substances (suspension of CB, CL, extracts of C6H14 and CCl4 from CL), dried in a thermostat to a constant mass at 37 °C, placed in sterile Petri dishes and moistened with distilled water so that the subsequent water drop is not absorbed into the paper. Thus, the humidity of the filter was maintained at the highest possible level, daily adding the necessary amount of water to the full moisture capacity with the help of a Pasteur pipette. 20 caryopsis were placed on humidified filters in each Petri dish. The experiment was carried out in three repetitions for each option. On the third and fifth days, the height of the plantule and the length of the longest root were determined. Growth index (I) was used to assess the effect of the substances on the development of barley seedlings of the Emerald variety (Андреева, Кожевин, 2014):
I = (R + P) D
where I – is the growth index;
R + P – total values of the lengths of roots and plantules, respectively, cm;
D – is the percentage of germinated grains, %.
Study of the effect of exometabolites of soil cyanobacteria N. paludosum on the growth and development of 7-day-old barley plants without plantules
The filter paper was placed in Petri dishes and moistened with sterile distilled water to the full moisture capacity of the filter. The humidity of the filter was maintained by daily application of water to the filter with a Pasteur pipette. The barley grains treated for five minutes with 75% ethyl alcohol were laid on the moistened filters. On the 7th day of germination, the plantule were separated from the caryopsis with tweezers to exclude the nutrition of plants with substances contained in the endosperm of the caryopsis.
The plantule without caryopsis were placed in Petri dishes on filter paper (pre-impregnated with a solution of CiSO4; suspension of CB; suspension of CB + CuSO4; CL; CL + CuSO4; C6H14; C6H14 + CuSO4; CCl4; CCl4 + CuSO4, dried to a constant mass and before the layout of plants moistened with sterile water). In the control version, the filters were moistened with distilled water. In variants with copper sulfate (II), a concentration of Su2+ 3 mg/kg of the substrate was created, which corresponds to the maximum permissible concentration of mobile forms of copper in the soil. Each variant had 30 plants (n = 3). On the 3rd day of the exposure of plantules without caryopsis, the height of the plantules and the length of the longest root were determined. The intensity of lipid peroxidation (LPO) in barley was estimated by the accumulation of malondialdehyde (MDA), which is formed in plant tissues in the process of LPO in reaction with thiobarbituric acid (Лукаткин, 2002). MDA content was assessed photometrically using SPEKOL 1300 spectrophotometer (Analytik Jena, Germany). The content of boroficin (Merken, Beecher, 2000) and copper in plantules was determined (Сборник методик..., 2004). Throughout the experiment, the dishes with plants were in the climatostat with a night temperature +12-13 °C, and the daytime temperature +21-23 °C.
Statistical processing and plotting were performed in Excel 2002 for Windows. The figures show arithmetic averages and standard error. The reliability of the differences between the two averages was assessed using the Student's t-test.
Results
Exometabolite composition of culture liquid of cyanobacteria N. paludosum. In the process of the CB life, a number of biologically active substances are formed and released into the environment. In the conditions of water culture, exometabolites of CB accumulate in the CL. It was found that extracts from CL and, accordingly, the CL itself contain substances such as phytoin (0.5–0.8 mcg/dm3), phytofluin (0.6–0.8 mcg/dm3), N-acetylglucosamine (about 2.5 mcg/dm3), peptidoglycan murein, antioxidants: lycopene (190-195 mcg/dm3 – organic extracts and CL, 240-250 mcg/dm3 – in suspension CB) and lutein (20-25 mcg/dm3). Hormones, precursors of gibberellins (kauren), vitamin A and provitamins were found. It should be noted that in general, the compositions of cyanobacterial suspension, CL and extracts from it do not differ significantly in the number of certain organic substances. The only distinctive feature is the high content of lycopene in the suspension of CB. Theoretically, most of these substances can have a positive effect on the development of plants. N. muscorum was previously found to produce the cyanopeptide boroficin, which has antimicrobial activity (Banker, Carmeli, 1998; Swain et al., 2017). Probably, just it can determine the antifungal activity of the studied substances. In our experiment, the concentration of this cyanopeptide during its extraction in the substance:extractant ratio, equal to 1:1, in all types of substances was in the range of 0.05–0.06 µg/dm3. The presence of boroficin in the CB genus Nostoc, in particular in Nostoc linkia and N. spongiaeforme, was noted in 1994 (Hemscheidt et al., 1994). This cyanopeptide is biologically active, so it is necessary to investigate the possibility of its migration into plants.
By the fifth day, the stimulating effect of CL is leveled, although in other variants, the height of the plantule and the length of the root still exceed these indicators in the control by 10-15 % and 33-37 %, respectively (Fig. 1B).
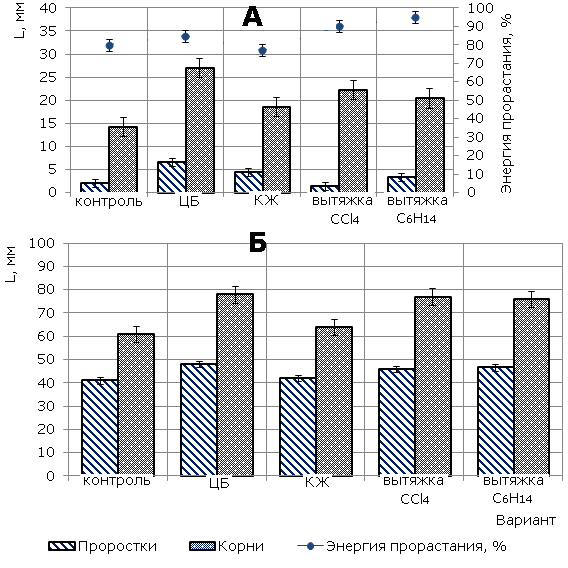
Fig.1. The influence of cyanobacterium N.Paludosum and its exometabolites on morphometric parameters of barley of Izumrud variety (A - the 3rd day, B - the 5th day), «*» - results are reliably different from the controls at P>0.95
Using the growth index indicator (Fig. 2) to illustrate the observed effects, the integral characteristic of the influence of the studied substances on the growth and development of barley plants was revealed. The use of cyanobacterial suspension, extracts of C6H14 and CCl4 in general has a growth-stimulating effect, which was revealed on 3-day-old plantules. To the fifth day of the experiment, this trend persists, but it is no longer possible to reliably judge about the stimulation of growth.
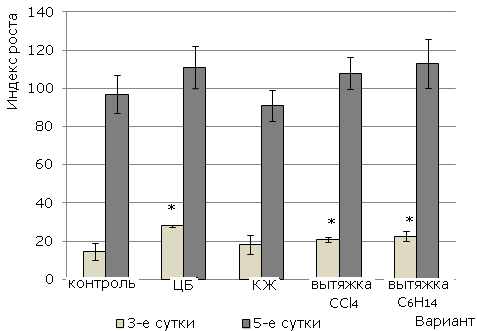
Fig. 2. The effect of cyanobacterium N. paludosum and its exometabolites on the plant growth index of barley Izumrud variety. «*» - results are reliably different from controls at P>95 (n=60)
Thus, it is established that the complex of components of all substances stimulates root growth in the early stages of barley development. At the same time, the cyanobacterial suspension has the greatest growth-stimulating effect.
Assessment of the influence of the studied substances on the growth and development of barley plants, freed from grains. The effect of cyanobacterial metabolites on barley growth was studied in the experiment with 7-day-old plantules with removed caryopsis. 7-day-old plants without caryopsis were transferred to Petri dishes on filter paper pretreated with the studied substances. After 3-day exposure, morphometric parameters, the content of boroficin in plantules, copper – in plantules and roots were determined.
The height of the plantules. The greatest growth-stimulating effect in relation to the height of plantules was established in the variants with C6H14 extract (95 ± 3 mm) and CB suspension (94 ± 5 mm) versus 90 ± 4 mm in the control. However, there was no significant difference between the values of this parameter in all variants without the addition of copper (II) sulfate (Fig. 3).
The length of the roots. The cultivation of 7-day-old barley plants without caryopsis on medium containing CB and their metabolites led to inhibition of root system growth (see Fig. 3). This phenomenon can be considered as a reaction to stress, aimed at adapting plants to the conditions of the growing environment, or the absence of the need to increase the linear size of the roots, providing a full nutrition of the plant. In the variant where metabolites were absent, copper ions (II) in an amount equal to 3 mg / kg of the substrate caused a significant lag in root growth compared to the control.
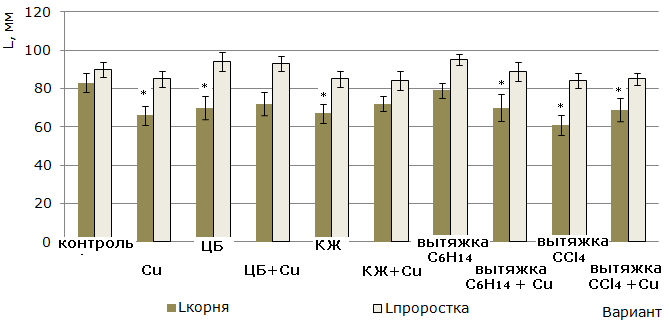
Fig. 3. The effect of N. paludosum, its exometabolites and copper on the growth of barley seedlings after the removal of grains. «*» - results are reliably different from controls at P>0.95 (n=30)
Effect of cyanobacteria cultural liquid components on copper accumulation by barley roots and plantules. The detection of copper in plants without the addition of metal salt is a consequence of the fact that Cu is a trace element that is a part of the grains in the natural state. The average copper content in plants, according to Vinogradov, is 2 mcg/g (Ягодин и др., 2002), the permissible residual amount is 10 mcg/g (Найштейн и др., 1987). In these parameters, the value of the copper includes not only of the control variant, but also of the variants with СL, C6H14 and CCl4 extracts (Fig. 4). In other variants, the well-being with the content of copper in the plantules slightly increased the amount of copper in the roots. In this case, the addition of copper sulfate (II) leads to a tendency to increase the accumulation of metal.

Fig. 4. The content of copper in the seedlings and roots of 10-day-old barley of Izumrud variety, after the removal of grains (n=30; P=0.95)
The influence of components of the culture fluid on boroficin accumulation in seedlings of barley. On the one hand, cyanopeptide boroficin can cause poisoning in mammals, thus playing a negative role, on the other – has antimicrobial properties in relation to phytopathogens. The interest shown to peptides in recent years is connected both with the possibility of using the genes of microorganisms responsible for the production of substances to create sustainable forms of agricultural plants, and with the prospects of their use for the development of new generation of drugs (Кокшарова, 2010; Одинцова и др., 2012).
It was found that boroficin accumulates in seedlings when growing barley on CB suspension and CCl4 extract from CL in an amount equal to 0.0008-0.001 mcg / g of dry weight. In sprouts of other variants, it is not found. In this case, boroficin is present in almost the same amount (0.04 µg / dm3) in all substances. It is logical that such a feature is associated with a complex of factors both from the side of the plant and by the strength of the complex of substances on which the plants were grown. There is no definite answer yet.
The influence of exometabolites on lipid peroxidation in the leaves of barley. Lipid peroxidation (LP) – oxidative degradation of lipids in plants occurs mainly under the action of free radicals and is one of the symptoms of oxidative stress in plant cells (Apel, Hirt, 2004). Normally, LP is maintained at a certain level due to the effective operation of antioxidant defense systems (antioxidant enzymes, substances with antioxidant properties) (Blokhina et al., 2003). In stressful conditions, changes in the intensity of the LP processes can indicate both pathological conditions (Лелевич, 2009) and adaptation processes.
Cultivation of barley plantules on the test substrates led to a significant, compared with the control (water), decrease in the accumulation of the product of LP – malon dialdehyde (MDA) in the leaf cells (Fig. 5). Low intensity of LP processes was observed in the variant with the action of copper salts, which may be associated with the course of adaptive rearrangements in cells. It is known that immediately after the action of the stress factor there is a significant activation of antioxidant enzymes and the accumulation of substances with antioxidant properties in the cells. All this leads to a decrease in the level of reactive oxygen forms, which affects the speed of the LP processes in cells. It is known that organophosphorus xenobiotic – methylphosphonic acid and CB – N. muscorum cause a decrease in the level of MDA in barley leaf cells (Коваль, Огородникова, 2014), low temperatures can also lead to a decrease in the level of MDA in tobacco leaf cells (Попов и др., 2010). The decrease in the intensity of LP in barley cells, grown on the test substrates, may be due to the action of exometabolites of the CB, which contain substances with pronounced antioxidant properties – lycopene, lutein, vitamin A, etc.

Fig.5. The content of malonic dialdehyde in the leaf cells of 10-days-old seedlings of barley of Izumrud variety after the removal of grains (raw biomass equivalent)
Conclusions
In the culture liquid of the cyanobacterium N. paludosum, HPLC method detected substances that may have biological activity (lycopene, lutein-antioxidants, vitamin A and provitamins, precursors of gibberellins), contributing to the growth of barley. The cyanopeptide boroficin was also found in the culture liquid. Cyanobacteria N. paludosum 18 and its exometabolites largely affect the development of barley Izumrud varieties in the first three days of its growth, providing a stimulating effect on the linear growth of aboveground organs and roots. The study showed that the presence of cyanobacteria N. paludosum and its exometabolites leads not only to a growth-stimulating effect, but also to a decrease in the intensity of lipid peroxidation processes in plant cells.
References
Andreeva O. A. Kozhevin P. A. Optimization of the natural community of soil microorganisms as a way to create microbial fertilizers, Vestnik Moskovskogo universiteta. Ser. 17: Pochvovedenie. 2014. No. 4. P. 42–45.
Andreyuk E. I. Kopteva Zh. P. Zanina V. A. Cyanobacteria. Kiev: Naukova dumka, 1990. 200 p.
Apel K., Hirt H. Reactive Oxygen Species: Metabolism, Oxidative Stress, and Signal Transduction, Annu. Rev. Plant Biol. 2004. Vol. 55. P. 373–399. DOI: 10.1146/annurev.arplant.55.031903.141701
Banker R., Carmeli S. Tenuecyclamides A-D, cyclic hexapeptides from the cyanobacterium Nostoc spongiaeforme var. tenue, Journal of Natural Products. 1998. Vol. 61. Issue 10. P. 1248–1251. DOI: 10.1021/np980138j
Belyh O. I. Gladkih A. S. Sorokovikova E. G. Tihonova I. V. Potapov S. A. Butina T. V. Saxitoxin-producing cyanobacteria in Lake Baikal, Sibirskiy ekologicheskiy zhurnal. 2015. No. 2. P. 229–237.
Blokhina O., Virolainen E., Fagerstedt K. V. Antioxidants, Oxidative Damage and Oxygen Deprivation Stress: a Review, Annals of Botany. 2003. Vol. 91. R. 179–194. DOI: 10.1093/aob/mcf118
Chemical encyclopedia, Pod red. N. P. Zefirova M.: Bol'shaya Rossiyskaya enciklopediya, 1998. T. 5. 783 p.
Collection of methods for measuring the concentration of copper, cadmium, zinc, bismuth, manganese and nickel ions using voltammetry using the Ecotest-VA volt-ampere analyzer. M.: OOO «Ekoniks-Ekspert», 2004. 61 p.
Domracheva L. I. Kondakova L. V. Popov L. B. Zykova Yu. N. Bioremediation capabilities of soil cyanobacteria (review), Teoreticheskaya i prikladnaya ekologiya. 2009. No. 1. P. 8–17.
Domracheva L. I. "Flowering" of the soil and the patterns of its development. Syktyvkar, 2005. 336 p.
Fokina A. I. Gornostaeva E. A. Ogorodnikova S. Yu. Zykova Yu. N. Domracheva L. I. Kondakova L. V. Adaptation potential of naturally occurring cynaobacterial biofilms dominated by phormidium, Sibirskiy ekologicheskiy zhurnal. 2015. No. 2. P. 842–851. DOI: 10.15372/SEJ20150604
Hemscheidt T., Puglisi M. P., Larsen L. K., Patterson G. M. L., Moore R. E., Rios J. L., Clardy J. Structure and biosynthesis of borophycin, a new boeseken complex of boric acid from a marine strain of the blue-green alga Nostoc linckia, J. Org. Chem. 1994. Vol. 59. P. 3467–3471.
Ivkova A. V. Petrova S. N. Qualitative analysis of hexane extracts of wild rose leaves, Izvestiya vuzov. Prikladnaya biohimiya i biotehnologiya. 2012. No. 2 (3). P. 158–159.
Koksharova O. A. Application of molecular genetics and microbiology in ecology and biotechnology of cyanobacteria, Mikrobiologiya. 2010. T. 79. No. 6. P. 734–747.
Koval' E. V. Ogorodnikova S. Yu. The effect of cyanobacterium Nostoc muscorum on the resistance of barley plants to the action of methylphosphonic acid, Teoreticheskaya i prikladnaya ekologiya. 2014. No. 2. P. 61–66.
Lelevich V. V. Biological Chemistry. Grodno: GrGMU, 2009. 275 p.
Lukatkin A. S. Cold damage to heat-loving plants and oxidative stress. Saransk: Izd-vo Mordov. un-ta, 2002. 208 p.
Merken H. M., Beecher G. R. Liquid chromatographic method for the separation and quantification of prominent flavonoid aglycones, Journal of Chromatography A. 2000. Vol. 897. Issues 1–2. R. 177–184. DOI: 10.1016/s0021-9673(00)00826-8
Nayshteyn S. Ya. Merenyuk G. V. Chegrinec G. Ya. Environmental health and fertilizer use. Kishinev: Shtiinca, 1987. 143 p.
Odincova T. I. Korostyleva T. V. Utkina L. L. Andreev Ya. A. Slavohotova A. A. Istomina E. A. Puhal'skiy V. A. Egorov C. A. Wheat antimicrobial peptides, Vavilovskiy zhurnal genetiki i selekcii. 2012. T. 16. No. 1. P. 107–115.
Ogorodnikova S. Yu. Zykova Yu. N. Berezin G. I. Domracheva L. I. Kalinin A. A. Comprehensive assessment of cyanobacteria under the influence of various pollutants, Teoreticheskaya i prikladnaya ekologiya. 2010. No. 3. P. 47–51.
Popov V. N. Antipina O. V. Trunova T. I. Lipid peroxidation during low-temperature adaptation of the leaves and roots of thermophilic tobacco plants, Fiziologiya rasteniy. 2010. T. 57. No. 1. P. 153–156.
Rott E., Pentecost A., Mareš J. Introduction: Recent developments in cyanobacterial research with special reference to aquatic habitats, molecular ecology and phylogenetic taxonomy, Hydrobiologia. 2018. Vol. 811. Issue 1. P. 1–6. DOI: 10.1007/s10750-017-3468-9
Rumyancev V. A. Kryukov L. N. Features of the nature of cyanobacteria, Zdorov'e naseleniya i sreda obitaniya. 2012. No. 2. P. 221–227.
Sakevich A. I. Freshwater Algae Exometabolites. Kiev: Naukova dumka, 1985. 199 p.
Sirenko L. A. Kozickaya V. N. Biologically active substances algae and water quality. Kiev: Naukova dumka, 1988. 256 p.
Swain S. S., Paidesetty S. K., Padhy N. R. Antibacterial, antifungal and antimycobacterial compounds from cyanobacteria, Biomedicine & Pharmacotherapy. 2017. June. Vol. 90. P. 760–776. DOI: 10.1016, j. biopha.2017.04.030
Voloshko L., Safronova T., Pljusch A., Titova N., Kopecky J., Hrouzek P., Drabkova V. Toxins and other bioactive compounds produced by cyanobacteria in Lake Ladoga, Estonian Journal of Ecology. 2008. No 2. P. 100–110. DOI: 10.3176/eco.2008.2.02
Wejnerowski Ł., Rzymski P., Kokociński M., Meriluoto J. The structure and toxicity of winter cyanobacterial bloom in a eutrophic lake of the temperate zone, Ecotoxicology. 2018. Vol. 27. Issue 6. P. 752–760. DOI: 10.1007, s10646-018-1957-X.
Yagodin B. A. Zhukov Yu. P. Kobzarenko V. I. Agrochemistry, Pod red. B. A. Yagodina. M.: Kolos, 2002. 584 p.
Řezanka T., Dembitsky V. M. Metabolites produced by cyanobacteria belonging to several species of the family Nostocaceae, Folia Microbiologica. 2006. Vol. 51. Issue 3. P. 159–182. DOI: 10.1007/BF02932119





 © 2011 - 2026
© 2011 - 2026