Introduction
Higher aquatic vegetation has a significant impact on species structure and abundance of zooplankton (Семенченко, Разлуцкий, 2009; Семенченко и др., 2013; Курбатова и др., 2018). Zooplanktocenoses of the macrophyte thickets are characterized by high species richness and quantitative development (Крылов, 2005; Лобуничева, 2008). The species structure of zooplankton communities in the coastal zone of water bodies is determined mainly by the intensity of macrophytes development (Kuczynska-Kippen, 2006), as well as their morphological structure (Семенченко, Разлуцкий,, 2009; Jeong et al., 2014). Submerged aquatic plants with greater biomass form a high heterogeneity of habitats, that causes an increase in the diversity and density of zooplankton (Spoljar et al., 2012; Choi et al., 2014). At the same time, some macrophytes (candock, bladderwort) have a depressing effect on zooplankton (Зимбалевская и др., 1987; Курбатова и др., 2012; Зайцева и др., 2014). In addition, the specific physicochemical conditions and trophic relationships formed in macrophyte thickets with different morphological structure, determine differences in the species structure of zooplankton communities (Курбатова и др., 2018).
To date, zooplankton thickets of higher aquatic plants of lowland rivers is studied extremely weak. In the literature there is evidence that zooplankton of coastal-aquatic macrophyte thickets of rivers are quantitatively more developed in comparison with an ungrown medial part (Крылов, 2005). In addition, the greatest quantitative development of macrophyte thickets zooplankton within the zone of river water retention was noted for the mouth of the small river-tributary of the plain reservoir (Столбунова, 2011). In the confluence zones of unregulated rivers, the structure of zooplankton depends on flow rates and degree of overgrowing by higher aquatic vegetation (Болотов и др., 2012). Generally higher aquatic vegetation has a significant effect on spatial distribution of zooplankton communities in areas of lowland rivers differing in morphometric characteristics (Гаврилко и др., 2018).
Special attention should be paid to the study of zooplanktocenoses of the confluence of rivers and lakes, where transitional zones are formed in which the river speed decreases, that favorable affects the development of higher aquatic vegetation in ripal. Areas of confluence of different types of water objects determine the structural and functional organization of hydrobionts communities of recipient objects (Болотов и др., 2012). In domestic literature there is practically no information on the species structure of zooplankton communities of different types of macrophytes in the contact zones of river and lake waters. One of such zones is the confluence section of the Serezha river and the Velikoye lake, whose coastal area is occupied by heterogeneous plant associations.
Serezha River – the right tributary of the second order of the Oka river. Its length is 196 km, basin area – 2730 km2. The river is characterized by winding, weakly branched sandy channel with stony rifts. The river floodplain is covered with forest, shrubs, swampy in places. Midstream forms a system of karst lakes (Velikoye, Glubokoye, Parovoye, etc.) (Природа ..., 1974).
Information about zooplankton of. Serezha riv. is represented in a number of works (Шурганова и др., 2012; Ильин и др., 2015, 2016; Ильин, 2016). However, the study of zooplankton in the thickets of higher aquatic vegetation of the river was held for the first time.
The goal of this work is to characterize the species structure and spatial distribution of the zooplankton communities of medial and thickets of higher aquatic vegetation in the Seryozha river in the confluence of the Velikoye lake.
Materials
Studies of zooplankton were conducted in the middle flow of the Seryozha river (Arzamas district, Nizhny Novgorod region) at its confluence with the Velikoye lake (N 55.658543, E 43.596692) in mid-July 2016. Sampling was carried out on a section of 1.3 km long before flowing into the lake, as well as in the Velikoye lake on the plot, after the confluence of the Serezha river (Fig. 1).
The macrophyte thickets of different morphological structures were selected: candock, Nuphar lutea (L.) Sm., water horsetail Equisetum fluviatile L., Sm., Canadian pondweed, Elodea canadensis Michx., crab's-claw, Stratiotes aloides L., Old-World arrowhead, Sagittaria sagittifolia L., shining pondweed, Potamogeton lucens L. All these biotopes are located in the river’s ripal, the medial part of which was characterized by a significant flow velocity (0.3–0.35 m / s). In the in the coast of the river, in the backwater area of the lake, there was a biotope of the batter dock, Potamogeton natans (L.).
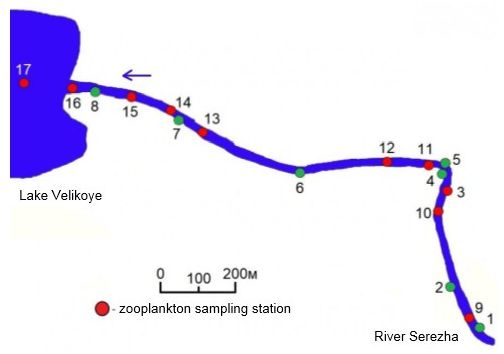
Fig. 1. Sceme of location of zooplankton sampling stations on the river Serezha in the zone of its influx into lake Velikoye. The stations in the macrophyte thickets are marked in green, in open water - in red. 1 – biotop of Nuphar lutea; 2 – biotop of Equisetum fluviatile; 3 – open coastal area; 4 – biotop of Elodea canadensis; 5 – biotop of Stratiotes aloides; 6 – biotop of Sagittaria sagittifolia; 7 – biotop of Potamogeton lucens; 8 – biotop of Potamogeton natans; 9–12 – medial of the river area; 13–16 – medial of the backwater zone, 17 – lake Velikoye
Methods
Zooplankton samples in the thickets of higher aquatic plants were taken with a measuring bucket, by filtering 50 liters of water through a plankton net (nylon sieve with a mesh of 70 microns) and fixed with 4 % formalin solution. In the medial zone of the river and in the lake, samples were taken by a Jedi’s net, by straining a column of water from the bottom to the surface. 3 zooplankton samples were taken in each thicket biotope. Processing of the material was carried out by conventional methods (Методические рекомендации..., 1982). Identification of zooplankton species was carried out using the key (Кутикова, 1970; Определитель..., 2010).
In parallel with sampling, measurements of depth, transparency, temperature, pH, conductivity of water, concentration of dissolved oxygen, flow rate, percentage of projective cover by plants of the biotope were carried out.
The similarity of zooplankton species composition was assessed on the basis of the Sörensen’s coefficient (Шнитников и др., 2003). The dominant species of zooplankton were identified according to the Paliy-Kownacki index of dominancy (Шнитников и др., 2003). Zooplankton samples were classified using cluster analysis based on the similarity of the species structure, the cosine of the angle between the vectors of samples in the multidimensional space of species numbers was used as a measure of similarity (Шурганова и др., 2003; Шурганова, 2007). Cluster analysis was carried out on the basis of calculations of the distance between groups of samples (clusters) by the mean connection method. To select the optimal number of clusters during the clustering of zooplankton samples, the analysis of silhouettes and analysis of the Mantel’s correlation coefficients were performed (Legendre, Legendre, 2012; Якимова и др., 2016). The data ordination was performed using redundancy analysis (RDA) (Legendre, Legendre, 2012; Шнитников, Розенберг, 2013). All calculations were performed in R environment (R Core Team, 2015).
The studied section of the river was characterized by neutral and slightly alkaline reaction of the water (pH 7.23-8.28). The electrical conductivity varied within 165.7-219.0 мкСм/cm. High concentrations of dissolved oxygen (9.54–12.58 mg/l) were observed in all biotopes, with the exception of water soldier and equisetum thickets (7.51–7.85 mg/l). All plant associations were characterized by the dominance of one plant species, which occupied more than 60 % of the projective cover in the biotope.
Results
In the zooplankton of the studied area of the Serezha river, 120 species were identified, 65 of which belonged to Rotifera, 39 – to Cladocera, 16 – to Copepoda. Among the identified species rotifer Kellicottia bostoniensis (Rousselet, 1908) was found – a invading species of the North American origin. Rotifers have been observed only at medial stations and open coastal areas, and have not been found in thicket biotopes.
A cluster dendrogram was constructed on the basis of the Sörensen’s coefficient of species composition similarity (Fig. 2). Zooplankton sampling stations groupe into two distinct clusters. The first cluster included stations from coastal biotopes (1-8), with the station No. 3, located in the open coast, being the farthest from the thicket samples. The second cluster form the stations of the medial zone of the river (9-16) and the lake (17).
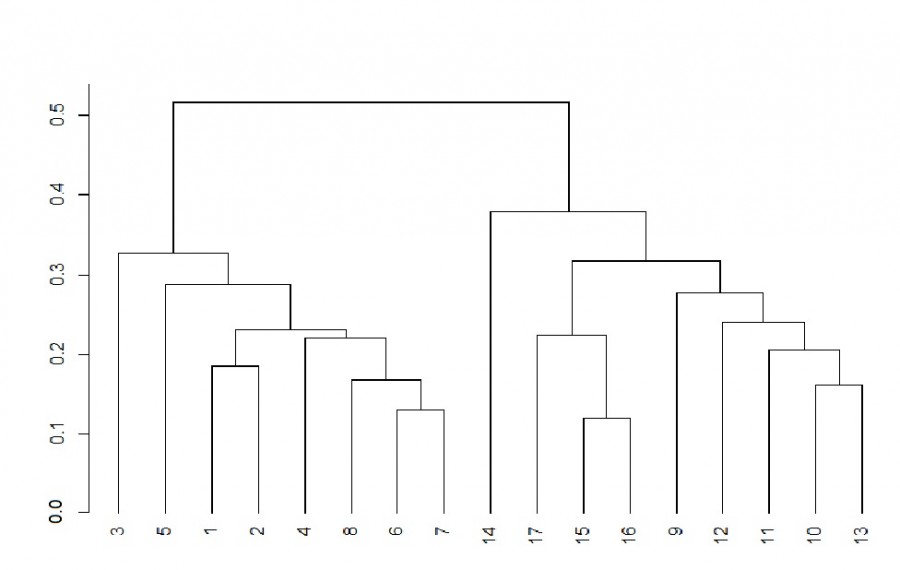
Fig. 2. Dendrogram of hierarchical clustering of zooplankton samples based on the Sörensen coefficient. Axis represents the joining distance. Description of sampling stations as in Fig. 1
The results of the dendrogram analysis (see Fig. 2) indicate differences in the species composition of zooplankton of the medial and coastal zones of the river. The largest number of zooplankton species among all coastal biotopes was typical for water soldier thickets (80 species) (table. 1), and the smallest – for the biotope of the open coast (45 species). At the same time, the number of species in the medial zone of the river was 42, which was relatively lower than in the thicket biotopes. Significant branching and the greater density of water soldier contributed to the formation of high species richness of zooplankton.
Table 1. Indicators of species structure and quantitative development of zooplankton communities
| Station | Biotope | Dominant | D | S |
N, thous.
spec./m3 |
N, % Rot:Cla:Cop | B, g/m3 | B, % Rot:Cla:Cop |
| 1 | Candock |
Conochiloides coenobasis
Keratella cochlearis Hexarthra mira |
33.3 16.6 15.8 | 61 | 755±183.9 | 84:5:11 | 1.31±0.23 | 54:9:37 |
| 2 | Swamp hostail |
Copepodit Cyclopoida
Ceriodaphnia pulchella Thermocyclops oithonoides Conochiloides coenobasis |
17.7 13.6 13.4 10.3 | 65 | 579±299.7 | 223:34:43 | 7.31±5.31 | 2:44:54 |
| 3 | Open coast |
Nauplii Copepoda
Conochiloides coenobasis Hexarthra mira |
20.6 17.2 13.5 | 45 | 133.9±38.9 | 63:10:27 | 0.23±0.02 | 38:20:42 |
| 4 |
Canadian
pondweed |
Ceriodaphnia pulchella
Ceriodaphnia quadrangula Thermocyclops crassus Diaphanosoma brachyurum |
26.3 17.1 11.1 10.1 | 61 | 872.4±89.8 | 1:64:35 | 22.82±5.96 | 1:79:20 |
| 5 |
Water
soldier |
Conochilus unicornis
Copepodit Cyclopoida Nauplii Copepoda |
29.5 25.1 16.3 | 80 | 343.2±21.3 | 36:20:44 | 2.31±0.22 | 3:62:35 |
| 6 |
Old-World
arrowhead |
Diaphanosoma brachyurum
Ceriodaphnia pulchella Copepodit Cyclopoida |
26.2 15.1 12.6 | 65 | 682.2±327.8 | 5:58:37 | 13.55±2.03 | 1:78:21 |
| 7 |
Cornstalk
weed |
Nauplii Copepoda
Copepodit Cyclopoida Conochiloides coenobasis |
22.4 20.3 12.8 | 62 | 111.7±25.3 | 34:22:44 | 1.49±0.67 | 4:84:12 |
| 8 | Batter dock |
Nauplii Copepoda
Polyarthra euryptera |
23.7 13.5 | 73 | 543.2±92.3 | 53:13:34 | 2.02±0.31 | 29:40:31 |
| 9-12 | Medial river zone without thickets |
Nauplii Copepoda
Conochiloides coenobasis Copepodit Cyclopoida Keratella cochlearis |
19.4 17.0 16.2 10.3 | 42 | 179.1±114.4 | 56:7:37 | 0.74±0.41 | 43:12:45 |
| 13-16 | Medial, retaining zone |
Nauplii Copepoda
Bosmina longirostris Brachionus angularis Copepodit Cyclopoida |
41.8 14.2 11.8 11.5 | 41 | 728.2±157.8 | 32:15:53 | 2.18±0.83 | 31:18:51 |
| 17 | The lake, after the confluence of the river |
Asplanchna priodonta
Copepodit Cyclopoida |
20.8 14.9 | 29 | 278.8 | 58:121:30 | 3.55 | 36:18:51 |
Note. D – dominance index, S – the total number of species, N – total abundance, B – total biomass, Rot:Cla:Cop – the ratio of rotifers, cladocerans and copepods.
- Based on the analysis of the species structure of zooplankton communities, a dendrogram of hierarchical clustering was constructed (Fig. 3). Based on the analysis of the silhouette width and Mantel’s correlation coefficient, the dendrogram was divided into five clusters. Stations No 9-12, located in the river medial zone with a pronounced flow rate, were characterized by high similarity of species structure, which allowed them to be grouped into one cluster. This zooplankton community was dominated by nauplial and copepoditic stages (CI–CV) of copepod crustaceans, rotifers Conochiloides coenobasis Skorikov, 1914 and Keratella cochlearis (Gosse, 1851) (tab. 1). The average zooplankton number was 179.1 ± 114.4 thousand specimens/m3, and the biomass was 0.74±0.41 g/m3.
Stations No. 13-16, located in the lake retaining zone, had a similar zooplankton species structure (see Fig. 3), indicating the formation of a transitional zooplanktocenosis at this site. The younger age stages of copepod crustaceans were dominant here, as well as the Cladocera crustacean Bosmina longirostris (O. F. Müller, 1785) and the rotifer Brachionus angularis Gosse, 1851 (see table. 1). The number and biomass of zooplankton of transitional cenosis were higher than in the river section (728.2 ± 157.8 thousand specimens/m3 and 2.18 ± 0.83 g/m3). The station No 17 of lake Velikoye is showed separately on the dendrogram (see Fig. 3). The species structure of zooplankton in this area differed significantly from river communities. This station was dominated by rotifers Asplanchna priodonta and copepodite stages (CI–CV) of copepods. The number of zooplankton was 278.8 thousand specimens/m3, biomass-3.55 g/m3.
The species structure of zooplankton communities of coastal biotopes (stations 1-8) differed significantly among themselves. The most isolated on the dendrogram are biotopes of submerged macrophytes with a large projective cover (Elodea, water soldier) and biotopes of air-aquatic plants (horsetail and arrowhead) (see Fig. 3). The zooplankton communities in the thickets of plants with floating leaves are more gravitated to medial areas. Thus, the thickets of the yellow pondlily (station 1) were located in the coast of the river free flow. This zooplanktocenosis was dominated by rotifers characteristic of open water zones – Conochiloides coenobasis, Keratella cochlearis and Hexarthra mira (Hudson, 1871). However, the total abundance and biomass of zooplankton were significantly higher than in the medial zone. The thickets of the tenchweed (station 8) were located in the coastal zone of the contact of river and lake waters. Here the nauplial stages of copepods and the rotifer Polyarthra euryptera (Wierzejski, 1891), which prefers lake and pond conditions, prevailed. The biotope of the thickets of cornstalk weed (station 7) fell into one cluster with samples from the medial river zone (see Fig. 3). The low projective cover of plants, along with a significant flow rate, contributed to the formation of a similar species structure of zooplanktocenoses of these thickets and the medial zone. This cluster also includes the biotope of the open coast (station 3).
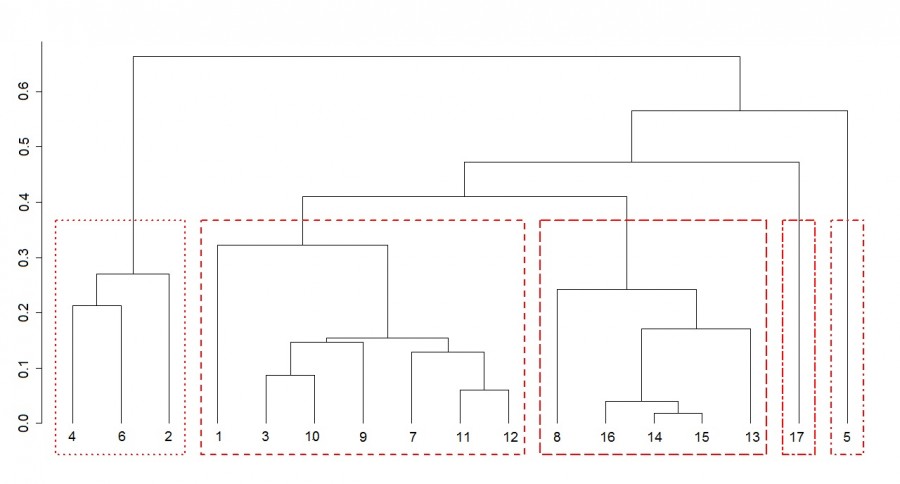
Fig. 3. Dendrogram of hierarchical clustering of zooplankton samples based on species structure. Axis shows the joining distance. The corresponding clusters are selected with the same line type. Description of sampling stations as in Fig. 1.
The zooplanktocenosis of the water soldier biotope (article 5) differed significantly from other thicket communities by the presence among the dominants the colonial rotifer Conochilus unicornis Rousselet, 1892. At the same time, the number and biomass of zooplankton were relatively low (see table. 1).
The zooplankton community of Elodea thickets (station 4), the Cladocera crustaceans Ceriodaphnia pulchella Sars, 1862, Ceriodaphnia quadrangula (O.F.Müller, 1785), Diaphanosoma brachyurum (Liévin, 1848), and the Copepoda crustacean Thermocyclops crassus (Fischer, 1853) dominated. Maximum values of zooplankton abundance (872.4 ± 89.8 thousand specimens/m3) and biomass (22.82 ± 0.96 g/m3) among all river biotopes were noted for this community. The zooplanktocenosis of Sagittarius thickets (st. 6) was also characterized by the predominance of cladocera crustaceans Diaphanosoma brachyurum and Ceriodaphnia pulchella. This allowed him to get into one cluster with zooplankton communities with the thickets of Elodea (see Fig. 3). There were also high values of zooplankton abundance and biomass (682.2 ± 327.8 thousand specimens/m3 and 13.55 ± 2.03 g/m3, respectively).
The composition of the dominants of zooplanktocenosis of horsetail thickets (st. 2) differed from the thickets of Elodea and Sagittarius by the presence of the crustacean Thermocyclops oithonoides (Sars, 1863) and the rotifer Conochiloides coenobasis. However, the dominance of Ceriodaphnia pulchella and copepodite stages (CI-CV) of copepod crustaceans caused the similarity of the species structure of zooplankton of these macrophyte thickets and the formation of a single cluster (see Fig. 3). The quantitative development of zooplankton in the thickets of horsetail was lower than in the thickets of Elodea and arrowhead (see table. 1).
- The ratio of numbers of studied taxonomic zooplankton groups of macrophyte thickets differed significantly. The predominance of rotifers in the total number of zooplankton was characteristic for plants with floating leaves (yellowcup, tenchweed) (see table. 1). The cladocerans dominated in the thickets of Elodea and arrowhead. The other biotopes were characterized by the predominance of copepod
Unlike zooplankton numbers, the most of the biomass of most thicket communities was composed of Cladocera crustaceans. The exception is the biotope of pondlily, where the predominance of rotifers was observed, as well as the biotope of horsetail, where copepoda crustaceans came to the fore (see table. 1).
To assess the dependence of the species structure of zooplankton communities on environmental factors, a model based on redundancy analysis (RDA) was constructed, taking into account the following abiotic and biotic parameters.
Statistical analysis of auxiliary models, which were constructed for each individual factor, showed that all factors, except the concentration of dissolved oxygen, had a significant influence p < 0.05 (table. 2). The complete RDA model explained 40.94 % (p < 0.001) of the total dispersion of the species zooplankton communities’ structure. The first two axes were statistically significant in the model (table. 3).
Table 2. Statistical analysis of the significance of explaining the variability of species structure for each individual factor
| Factor | Adjusted proportion of variance explained, % | The value of the Fisher criterion, F | p |
| The projective cover of plants | 21.0 | 5.26 | 0.001 |
| Temperature | 14.1 | 3.63 | 0.002 |
| Flow rate | 13.1 | 3.41 | 0.007 |
| Conductivity | 13.5 | 3.49 | 0.012 |
| pH | 12.2 | 3.22 | 0.007 |
| Transparency | 10.7 | 2.93 | 0.014 |
| Depth | 6.7 | 2.15 | 0.046 |
| The concentration of dissolved oxygen | 1.3 | 1.21 | 0.276 |
Table 3. Statistical analysis of model data based on the analysis of redundancy
| Model, axix | Adjusted proportion of variance explained, % | The value of the Fisher criterion, F | p |
| The complete model | 40.94 | 2.58 | 0.001 |
| Axis I | 19.18 | 8.47 | 0.002 |
| Axis II | 10.75 | 4.75 | 0.003 |
| Axis III | 3,94 | 1.74 | 0.138 |
| Axis IV | 3.12 | 1.38 | 0.224 |
The location of the stations on the ordination diagram, constructed according to the results of the redundancy analysis, is consistent with the results of the cluster analysis and indicates a different species structure of zooplankton communities of macrophyte thickets (Fig. 4). Stations corresponding to medial and thicket communities are located along the first (horizontal) axis of ordination, which significantly explains 19.18 % of the total dispersion of zooplankton (see table. 3). The position of the stations in the diagram along the second (vertical) axis, explaining the 10.75% variance (see table. 3), due to the influence of abiotic factors. The medial stations of the river section (9-12) are located along the correlated vectors of flow velocity and transparency (see Fig. 4). On the contrary, the stations located in the medial transition section of the river (13-16), as well as below the confluence of the river (17), are located along the pH vector. In the direction of pH increasing, the station number 8 of common floating pondweed thickets is situated, and it is located in the coastal transitional section of the river. Most of the other thicket stations are located along the vector of the projective cover of plants (see Fig. 4).
Discussion
The analysis of the obtained results makes it possible to distinguish in the medial of the Serezha river the areas occupied by zooplankton communities differing in species structure and indicators of quantitative development. Zooplanktocenosis of the river section with a pronounced flow rate was characterized by low quantitative development of zooplankton and numerical predominance of rotifers. The zooplankton community of the slow-flowing river section was characterized by an increase in the quantitative development of zooplankton compared to the river section. Here, the copepoda crustaceans dominate by number and biomass of zooplankton. The distinguishing of the transitional zooplanktocenosis of the Serezha river at the confluence with lake Velikoye is in good agreement with the results of previous studies (Ильин и др., 2015; Ильин, 2016).
The species structure of zooplankton communities of higher aquatic vegetation differed significantly between different biotopes and the medial zone. The thicket communities were characterized by the greatest species richness of zooplankton in the river. The highest quantitative zooplankton development was observed in the most densely closed macrophyte thickets (canadian pondweed and Old-World arrowhead) with a high projective cover (85-90%). The large volume of the submerged part of the macrophyte forms a high heterogeneity of the aquatic environment, which is a key factor in the mass development of zooplankton in the thickets. A number of authors also note that cladocera crustaceans achieve the greatest development in submerged macrophytes (Stefanidis, Parastergiadou, 2010; Bolduc et al., 2016; Курбатова и др., 2017), which provide high values of zooplankton biomass.
Thickets of plants with floating leaves (yellow cup, tenchweed) create a low heterogeneity of the aquatic environment, which leads to the proximity of these stations on the dendrogram (see Fig. 3) with river medial stations. These zooplanktocenoses were characterized by the predominance of rotifers in the total number of zooplankton. In the work of O. V. Mukhortova with co-authors (Mukchortova et al., 2015) also notes the similarity of the zooplankton of the yellow cup thickets with the open water zone.
In the dense thickets of the canadian pondweed and arrowhead the cladocerans dominated by the number of zooplankton. The basis of biomass of the majority of thicket communities of zooplankton was formed by cladocerian crustaceans.
The main factor determining the variability of the species structure of zooplankton communities was the projective cover of plants. The proximity of the thicket stations to the vector of the projective cover on the dendrogram (see Fig. 3) was determined by the density of thickets. Among the abiotic factors, the flow rate and pH of the water deserve special attention. The river section of the Serezha river is characterized by significant flow velocities (0.3 m / s) and neutral reaction of the environment (7.49–7.55). With the advance to the lake in the transitional section of the river, the flow rate fell to 0.1 m/s, and the pH of the water increased to 8.15. This is naturally reflected in the species structure of zooplankton communities and location stations on the ordinal dendrogram (see Fig. 4).
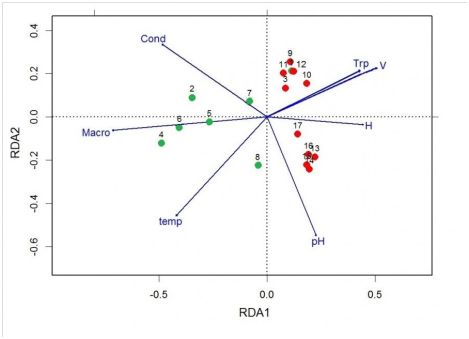
Fig. 4. Ordination dendrogramm plotted on the basis of redundancy analysis for zooplankton sampling, selected in the waters of the river Serezha. 1–17 – number of station, H – depth, temp – temperature, Cond – conductivity, Trp – transparency, pH – acidity, V – flow rate, Macro – plant cover. Description of stations as in Fig.1.
Thus, thickets of higher aquatic vegetation are the leading factor determining the species structure of zooplankton communities. They form zones of refugiums in the river forming favorable conditions for mass development of zooplankton and allowing to enrich plankton fauna of the river. If in the medial of the river the formation of plankton is determined mainly by the flow rate, then in ripali the main role is played by the higher aquatic vegetation (architectonics of macrophytes and density of thickets).
Conclusions
In the studied water area of the confluence of the Serezha river and Velikoye lake, zooplankton communities with different species structure, located in the medial of the river – the community of the river section and the community of the transition section are identified.
It was found that the species structure of zooplankton communities of different types of macrophyte thickets differed significantly between the thicket biotopes and the medial zone.
It was revealed that the greatest species richness of zooplankton in the river is concentrated in coastal thickets of higher aquatic vegetation. The greatest quantitative development of zooplankton communities is typical for the most dense thickets of macrophytes (canadian pondweed and Old-World arrowhead).
Analysis of the influence of environmental factors on the species structure of zooplanktocenoses showed that the largest part of the total dispersion was explained by such a parameter as the projective cover of plants.
References
Bolduc P., Bertolo A., Pinel-Alloul V. Does submerged aquatic vegetation shape zooplankton community structure and functional diversity? A test with a shallow fluvial lake system, Hydrobiologia. 2016. No. 778. R. 151–165.
Bolotov S. E. Cvetkov A. I. Krylov A. V. Zooplankton in the zones of unregulated rivers confluence, Biologiya vnutrennih vod. 2012. No. 2. P. 29–36.
Choi J. Y., Jeong K. S., La G. H., Joo G. J. Effect of removal of free-floating macrophytes on zooplankton habitat in shallow wetland, Knowledge and Management of Aquatic Ecosystems. 2014. Vol. 414 (11).
Gavrilko D. E. Kudrin I. A. Ruchkin D. S. Shurganova G. V. Influence of higher aquatic plants on the structure of zooplankton communities of a small river (on the example of the Vyunitsa River, Nizhny Novgorod), Materialy III mezhdunarodnoy konferencii «Aktual'nye problemy planktonologii». Kaliningrad: AtlantNIRO, 2018. P. 47–50.
Guidelines for the collection and processing of materials at hydrobiological research in freshwater bodies, Zooplankton i ego produkciya. L.: Gop. NII ozer. i rech. ryb. hoz-va, 1982. 33 p.
Il'in M. Yu. Kudrin I. A. Shurganova G. V. Bioindication of water bodies in specially protected natural areas of Nizhny Novgorod region based on the analysis of the species structure of zooplankton, Voda: himiya i ekologiya. 2016. No. 3. P. 25–33.
Il'in M. Yu. Shurganova G. V. Kuklina T. V. Kudrin I. A. Spatial distribution of zooplankton communities in the zone of contact between river and lake waters (on the example of the Serezha River and lake Velikoye in Nizhny Novgorod Volga region), Sovremennye problemy nauki i obrazovaniya. 2015. No. 6. URL: www.science–education.ru/130–23340.
Il'in M. Yu. Composition and structure of zooplankton communities of water bodies in specially protected natural territories (on the example of Nizhny Novgorod region): avtoref. dip. ... kand. biol. nauk. N. Novgorod, 2016. 27 p.
Jeong K. S., Choi J. Y., Jeong Kw. S. Influence of aquatic macrophytes on the interactions among aquatic organisms in shallow wetlands (Upo Wetland, South Korea), J. Ecol. Environ. 2014. Vol. 37 (4). P. 185–194.
Krylov A. V. Zooplankton of lowland small rivers. M.: Nauka, 2005. 263 p.
Kuczynska-Kippen N. Zooplankton structure in architecturally differentiated macrophyte habitats of shallow lakes in the Wielkopolska Region, Poland, International Journal of Oceanography and Hydrobiology. 2006. Vol 35. No. 2. P. 179–191.
Kurbatova S. A. Ershov I. Yu. Borisovskaya E. V. Influence of density of hydrophyte thickets on zooplankton, Biologiya vnutrennih vod. 2017. No. 1. P. 84–92
Kurbatova S. A. Lapteva N. A. Ershov I. Yu. Borisovskaya E. V. Background characteristics of the environment and the dynamics of plankton communities in ecosystems with hydrophytes, Povolzhskiy ekologicheskiy zhurnal. 2012. No. 1. P. 42–52.
Kurbatova S. A. Myl'nikova Z. M. Ershov I. Yu. Bykova S. N. Vinogradova O. G. Influence of aquatic plants of different ecological groups on the distribution and abundance of zooplankton, Sibirskiy ekologicheskiy zhurnal. No. 1. 2018. P. 56–66.
Kutikova L. A. Rotifers of the USSR fauna. L.: Nauka, 1970. 742 p.
Legendre P., Legendre L. Numerical ecology. Oxford: Elsevier, 2012. 990 p.
Lobunicheva E. V. Thicket zooplankton of some small lakes in Vologda region, Vodnye ekosistemy: troficheskie urovni i problemy podderzhaniya bioraznoobraziya: Materialy Vserop. konf. s mezhdunar. uchastiem «Vodnye i nazemnye ekosistemy: problemy i perspektivy issledovaniy» (Vologda, Rossiya, 24–28 noyabrya 2008 g.). Vologda, 2008. P. 188–192.
Mukchortova O., Bykova S., Tarasova N., Unkovskaya E., Bolotov S. Plankton Community in the Pelagic and Littoral Zones of the Overgrown Lake Beloe (Volzhsko-Kamskiy Biosphere Natural State Reserve, Republic of Tatarstan, Russian Federation), Journal of Siberian Federal University. Biology 1. 2015 (8). P. 66–84.
Nature of Gorky Region, Pod. red. N. V. Kuznecova. Gor'kiy: Volgo-Vyatskoe kn. izd-vo, 1974. 416 p.
R Core Team. R: A language and environment for statistical computing, R Core Team. 2015. URL: http://www.R–project.org.
Semenchenko V. P. Razluckiy V. I. Buseva Zh. P. Palash A. L. Zooplankton of the littoral zone of lakes of different types. Minsk: Belarup. navuka, 2013. 181 p.
Semenchenko V. P. Razluckiy V. I. Factors determining the daily distribution and movement of zooplankton in the littoral zone of freshwater lakes, Zhurnal Sibirskogo federal'nogo universitetata. Ser. Biologiya. 2009. No. 2. P. 191–225.
Shitikov V. K. Rozenberg G. S. Zinchenko T. D. Quantitative hydroecology: methods of system identification. Tol'yatti: IEVB RAN, 2003. 463 p.
Shitikov V. K. Rozenberg G. S. Randomization and bootstrap: statistical analysis in biology and ecology using R. Tol'yatti: Kassandra, 2013. 314 p.
Shurganova G. V. Cherepennikov V. V. Artel'nyy E. V. Dynamics of the spatial distribution of the main zooplankton cenoses of the Cheboksary Reservoir, Povolzhskiy ekologicheskiy zhurnal. 2003. No. 3. P. 297–304.
Shurganova G. V. Cherepennikov V. V. Tarbeev M. L. Maslova G. O. Species structure of the zooplankton of the river Serezha, Nizhny Novgorod region, Vestnik Nizhegorodskogo universiteta im. N. I. Lobachevskogo. 2012. No. 3 (1). P. 111–117.
Shurganova G. V. The dynamics of the species structure of zooplankton cenoses in the process of their formation and development (on the example of reservoirs of the middle Volga: Gorky and Cheboksary): Avtoref. dip. … d-ra biol. nauk. N. Novgorod, 2007. 48 p.
Spoljar M., Drazina T., Sargac J., Kralj Borojevic K., Zutinic P. Submerged macrophytes as a habitat for zooplankton development in two reservoirs of a flow-through system (Papuk Nature Park, Croatia), Ann. Limnol. Int. J. Lim. 2012. Vol. 48. P. 161–175.
Stefanidis K., Parastergiadou E. Influence of hydrophyte abundance on the spatial distribution of zooplankton in selected lakes in Greece, Hydrobiology. 2010. No. 656. P. 55–65.
Stolbunova V. N. Zooplankton thickets of macrophytes in the mouth area of a small river, Biologiya vnutrennih vod. 2011. No. 2. P. 35–42.
The determinant of zooplankton and zoobenthos of fresh waters of European Russia. Vol. 1. Zooplankton, Pod red. V. R. Alekseeva, P. Ya. Calolihina. M.: Tovarischestvo nauchnyh izdaniy KMK, 2010. 495 p.
Yakimov V. N. Shurganova G. V. Cherepennikov V. V. Kudrin I. A. Il'in M. Yu. Methods of comparative assessment of the results of cluster analysis of hydrobiocenoses structure (on the example of zooplankton communities of the Linda River, Nizhny Novgorod region), Biologiya vnutrennih vod. 2016. No. 2. P. 94–103.
Zayceva V. L. Filippov D. A. Lobunicheva E. V. Mihaylova A. A. Influence of Urticularia intermedia on the structure of communities of aquatic invertebrates in wetland reservoirs, Izvestiya Samarskogo nauchnogo centra RAN. 2014. T. 16. No. 5. P. 276–281.
Zimbalevskaya L. N. Pligin Yu. V. Horoshih L. A. Dolinskiy V. L. Sidorenko V. M. Levina O. V. Georgievskaya L. M. Revenko I. G. Goshovskaya G. A. Kozina S. Ya. Emel'yanov L. V. Levitskaya N. A. Structure and succession of the littoral biocenoses of the Dnieper reservoirs. Kiev: Nauk. dumka, 1987. 204 p.





 © 2011 - 2026
© 2011 - 2026