Introduction
Among the phytophagous insects that inhabit forests, most species have very low population densities, when the damage to forage plants is very rare or absent. But there is a group of species whose population size can change by 5-7 orders every few years. A large part of the forest insects with periodic changes in the number of populations refers to a group of coniferous- and leaf-eating insects and causes severe defoliation of forage plants, but rarely – their death. However, mass reproduction of coniferous- and leaf-eating insects and the weakening of the forest lead to the growth of stem pests-insects number that feed on bark and wood tissues. In Siberia, foci of stem pests are often formed after outbreaks of mass reproduction of the Dendrolimus sibiricus. Insects-stem pests accelerate the process of death of plants and stands in general.
The purpose of this work is to identify the spatial and temporal synchronization of the development of outbreaks of mass reproduction of xylophage insect species complex in the forests of the Krasnoyarsk region. This analysis is necessary to assess the risks of outbreaks and the optimization of the monitoring of xylophage insects that damage forest stands. Knowing the spatial relationship of outbreaks of a particular species of xylophage allows to assess the risk of outbreaks in other territories if a mass breeding site of this species is detected in a separate forest area. The knowledge of the temporal conjugation of the development of outbreaks of several xylophagous species in a separate forestry makes it possible to assess the risks of breeding foci of other xylophagous species when detecting outbreaks of mass reproduction of other species in this forestry.
Materials
The data on damage of forest plantations and information on forest pests were collected during ground surveys of current forest pathology forest plantations by the Center of forest protection of the Krasnoyarsk region in various forest areas during 2007-2015. In the process of forest pathology survey, the boundaries of forest damage, the accounting of the number of pests, assessment of insect damage to forest stands was carried out. The pathological examination of forests inhabited by stem pests was carried out by employees by visual inspection of weakened areas of the forest. The population of plantations with stem pests was estimated by the presence of shrunken and shrinking trees, by the withering of needles in the crown, the presence of drill flour on the bark, crawling beetles, and entrance and exit holes. An area where the number of trees inhabited by stem pests exceeds 10 % is considered to be a source of stem pests (Лесная энтомология, 2010).
In the course of forest pathology surveys in the forest Fund of the Krasnoyarsk territory, foci of mass reproduction of 15 species of xylophages were found, in particular such species of stem pests as Dicerca aenea L., Melanophila guttulata Gebl. (Coleoptera, Buprestidae), Monochamus urussovi Fisch., Monochamus sutor L., Monochamus galloprovincialis Ol., Saperda carcharias L. (Coleoptera, Cerambycidae), Xylechinus pilosus Ratz., Tomicus minor Hartig., Tomicus piniperda L., Dendroctonus micans Kug., Polygraphus proximus Blandf., Scolytus ratzeburgi Jans., Ips sexdentatus Boern., Ips subelongatus Motsch. Ips typographus L. (Coleoptera, Scolytidae).
In table. 1 the data on the area of stem pest foci in forest stands in the territory of forest districts of the Krasnoyarsk territory (unpublished materials of the annual reports of the forest protection Center Krasnoyarsk region) is shown.
Table 1. Areas (ha) of damage to forest stands by insects-xylophages in the territory of Krasnoyarsk Region
| № | Species of insect-
xylophages |
Forestry | Areas (ha) of insects-xylophages damage foci by years | |||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |||
| 1 | Polygraphus proximus | Achinskoye | 0 | 0 | 753.5 | 753.5 | 863.5 | 1009.4 | 1681.4 | 984.5 |
| 2 | Monochamus urussovi | Achinskoye | 0 | 13.7 | 13 | 13 | 13 | 13 | 13 | 13 |
| 3 | Monochamus urussovi | Balakhtinskoye | 0 | 41.5 | 35.3 | 33.2 | 0 | 0 | 0 | 0 |
| 4 | Tomicus piniperda | Bogotolskoye | 0 | 0 | 0 | 54 | 0 | 0 | 0 | 0 |
| 5 | Polygraphus proximus | Bogotolskoye | 0 | 0 | 474.6 | 474.6 | 474.6 | 474.6 | 143.3 | 115.3 |
| 6 | Xylechinus pilosus | Bogotolskoye | 0 | 54 | 54 | 54 | 54 | 54 | 0 | 0 |
| 7 | Monochamus urussovi | Bogotolskoye | 0 | 373.8 | 350.8 | 350.8 | 350.8 | 350.8 | 25 | 25 |
| 8 | Tomicus minor | Boguchanskoye | 10 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 9 | Monochamus sutor | Boguchanskoye | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 10 | Polygraphus proximus | Bolshemurtinskoye | 0 | 0 | 0 | 0 | 0 | 0 | 475 | 475 |
| 11 | Polygraphus proximus | Bolsheuluyskoye | 0 | 0 | 0 | 0 | 0 | 0 | 676.3 | 676.3 |
| 12 | Ips subelongatus | Borskoye | 50 | 17.5 | 17.5 | 17.5 | 0 | 0 | 0 | 0 |
| 13 | Monochamus urussovi | Verhne-Manskoye | 0 | 659 | 659 | 609 | 609 | 609 | 609 | 609 |
| 14 | Ips sexdentatus | Verhne-Manskoye | 20 | 20 | 66 | 979 | 979 | 979 | 933 | 933 |
| 15 | Dicerca aenea | Gremuchinskoye | 45 | 45 | 0 | 0 | 0 | 0 | 0 | 0 |
| 16 | Ips subelongatus | Gremuchinskoye | 41 | 41 | 41 | 5.4 | 5.4 | 188.1 | 78.4 | 30.2 |
| 17 | Ips sexdentatus | Gremuchinskoye | 423 | 213.6 | 213.6 | 213.6 | 213.6 | 213.6 | 213.6 | 213.6 |
| 18 | Tomicus minor | Gremuchinskoye | 298 | 297.8 | 297.8 | 23.1 | 15.2 | 15.2 | 15.2 | 80 |
| 19 | Tomicus piniperda | Gremuchinskoye | 39 | 25.1 | 25.1 | 0 | 23.1 | 259 | 121.3 | 48.6 |
| 20 | Monochamus sutor | Gremuchinskoye | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 21 | Melanophila guttulata | Gremuchinskoye | 0 | 0 | 45 | 45 | 45 | 45 | 45 | 96.3 |
| 22 | Saperda carcharias | Gremuchinskoye | 0 | 0 | 0 | 0 | 0 | 35 | 35 | 12.8 |
| 23 | Xylechinus pilosus | Gremuchinskoye | 0 | 0 | 0 | 15.2 | 0 | 0 | 0 | 0 |
| 24 | Monochamus urussovi | Gremuchinskoye | 0 | 3287.4 | 3279.6 | 3241.2 | 3241.2 | 3241.2 | 3203.2 | 3203.2 |
| 25 | Scolytus ratzeburgi | Dzerzhinskoye | 0 | 0 | 0 | 0 | 0 | 0 | 22 | 0 |
| 26 | Monochamus urussovi | Dzerzhinskoye | 0 | 0 | 0 | 0 | 0 | 2 | 2 | 2 |
| 27 | Tomicus minor | Divnogorskoye | 16 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 28 | Monochamus urussovi | Dolgomostovskoye | 0 | 95.3 | 95.3 | 205.3 | 110 | 110 | 110 | 110 |
| 29 | Ips subelongatus | Dolgomostovskoye | 24 | 24 | 24 | 24 | 0 | 0 | 0 | 0 |
| 30 | Scolytus ratzeburgi | Dolgomostovskoye | 1 | 0.7 | 0.7 | 0.7 | 0 | 0 | 0 | 0 |
| 31 | Polygraphus proximus | Emelyanovskoye | 0 | 0 | 0 | 0 | 0 | 0 | 109.2 | 1785 |
| 32 | Monochamus urussovi | Eniseyskoye | 0 | 10081 | 10144 | 1711 | 2227 | 2227 | 831 | 831 |
| 33 | Monochamus urussovi | Ermakovskoye | 0 | 3280.4 | 3280.4 | 3280.4 | 3280.4 | 3280.4 | 3140 | 3140 |
| 34 | Ips sexdentatus | Irbeyskoye | 206 | 206 | 206 | 206 | 206 | 206 | 206 | 206 |
| 35 | Tomicus piniperda | Irbeyskoye | 21 | 21 | 21 | 0 | 21 | 12 | 12 | 12 |
| 36 | Tomicus minor | Irbeyskoye | 0 | 0 | 0 | 21 | 0 | 0 | 0 | 0 |
| 37 | Monochamus urussovi | Irbeyskoye | 0 | 35508 | 35508 | 35508 | 35508 | 27250 | 26520 | 0 |
| 38 | Monochamus urussovi | Kazachinskoye | 0 | 7750 | 150 | 150 | 0 | 0 | 0 | 0 |
| 39 | Ips sexdentatus | Karatuzskoye | 0 | 0 | 0 | 0 | 0 | 295 | 295 | 295 |
| 40 | Monochamus urussovi | Karatuzskoye | 0 | 4065 | 4065 | 3434 | 3434 | 3567 | 1732 | 1732 |
| 41 | Ips sexdentatus | Kizirskoye | 0 | 0 | 0 | 5.8 | 5.8 | 5.8 | 5.8 | 5.8 |
| 42 | Monochamus urussovi | Kizirskoye | 0 | 798 | 2961.1 | 2669.3 | 2650.9 | 2496.2 | 2496.2 | 2048.2 |
| 43 | Ips subelongatus | Kodinskoye | 84 | 84 | 84 | 84 | 84 | 84 | 84 | 84 |
| 44 | Ips sexdentatus | Kodinskoye | 31 | 23.4 | 23.4 | 23.4 | 23.4 | 23.4 | 23.4 | 23.4 |
| 45 | Tomicus piniperda | Kodinskoye | 1580 | 1580 | 1580 | 0 | 1580 | 1580 | 1580 | 1529.8 |
| 46 | Scolytus ratzeburgi | Kodinskoye | 0 | 92 | 92 | 92 | 92 | 92 | 92 | 92 |
| 47 | Dicerca aenea | Kodinskoye | 0 | 360 | 0 | 0 | 0 | 0 | 0 | 0 |
| 48 | Melanophila guttulata | Kodinskoye | 0 | 0 | 360 | 360 | 360 | 360 | 360 | 360 |
| 49 | Tomicus minor | Kodinskoye | 0 | 0 | 0 | 1580 | 0 | 0 | 21.2 | 21.2 |
| 50 | Monochamus urussovi | Kodinskoye | 0 | 2391 | 2391 | 2391 | 2391 | 2391 | 2293 | 2578.9 |
| 51 | Polygraphus proximus | Kozulskoye | 0 | 0 | 724.5 | 884.2 | 887.5 | 505.4 | 1196.3 | 1174.6 |
| 52 | Monochamus urussovi | Kozulskoye | 0 | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 0 | 0 |
| 53 | Tomicus minor | Krasnoyarskoye | 30 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 54 | Tomicus piniperda | Krasnoyarskoye | 17 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 55 | Polygraphus proximus | Krasnoyarskoye | 0 | 0 | 0 | 0 | 0 | 0 | 366.3 | 366.3 |
| 56 | Monochamus urussovi | Krasnoyarskoye | 0 | 89.5 | 84 | 84 | 0 | 0 | 0 | 0 |
| 57 | Monochamus urussovi | Maganskoye | 0 | 83 | 83 | 82.5 | 82.5 | 82.5 | 82.5 | 82.5 |
| 58 | Scolytus ratzeburgi | Manzenskoye | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 59 | Ips subelongatus | Manzenskoye | 21 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 60 | Dendroctonus micans | Manzenskoye | 5 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 61 | Monochamus urussovi | Manzenskoye | 0 | 67.1 | 48.5 | 22 | 22 | 22 | 22 | 22 |
| 62 | Dicerca aenea | Manskoye | 123 | 123 | 103 | 54 | 54 | 54 | 8 | 8 |
| 63 | Ips sexdentatus | Manskoye | 978 | 1084.4 | 1025.9 | 1291 | 1305.9 | 1231.9 | 1181.9 | 1280.9 |
| 64 | Melanophila guttulata | Manskoye | 0 | 0 | 0 | 7 | 0 | 0 | 0 | 0 |
| 65 | Polygraphus proximus | Manskoye | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 5.1 |
| 66 | Monochamus urussovi | Manskoye | 0 | 574.2 | 571.4 | 598.1 | 583.2 | 583.2 | 583.2 | 583.2 |
| 67 | Monochamus sutor | Manskoye | 0 | 0 | 0 | 14.9 | 0 | 0 | 0 | 0 |
| 68 | Polygraphus proximus | Mininskoye | 0 | 0 | 0 | 0 | 0 | 0.4 | 707.22 | 749.1 |
| 69 | Monochamus urussovi | Mininskoye | 0 | 8.1 | 8.1 | 8.1 | 0 | 0 | 0 | 0 |
| 70 | Monochamus urussovi | Motyginskoye | 0 | 42143.6 | 40644.6 | 40361.6 | 40361.6 | 40361.6 | 35780.6 | 15342 |
| 71 | Ips typographus | Nazarovskoye | 27 | 16.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 72 | Polygraphus proximus | Nazarovskoye | 0 | 0 | 0 | 0 | 0 | 0 | 1522.4 | 1470.4 |
| 73 | Ips subelongatus | Nevonskoye | 4 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 | 4.2 |
| 74 | Tomicus minor | Nevonskoye | 119 | 98.9 | 86.3 | 21.4 | 49.9 | 49.9 | 161.9 | 123.9 |
| 75 | Tomicus piniperda | Nevonskoye | 39 | 39.4 | 39.4 | 0 | 21.4 | 21.4 | 21.4 | 21.4 |
| 76 | Scolytus ratzeburgi | Nevonskoye | 3 | 3 | 3 | 3 | 3 | 3 | 3 | 3 |
| 77 | Melanophila guttulata | Nevonskoye | 0 | 0 | 0 | 0 | 0 | 0 | 245.5 | 245.5 |
| 78 | Xylechinus pilosus | Nevonskoye | 0 | 0 | 0 | 49.9 | 0 | 55 | 55 | 55 |
| 79 | Monochamus urussovi | Nevonskoye | 0 | 104.9 | 104.9 | 104.9 | 104.9 | 104.9 | 137.9 | 137.9 |
| 80 | Ips sexdentatus | Pirovskoye | 87 | 87 | 87 | 87 | 87 | 87 | 87 | 70 |
| 81 | Ips typographus | Pirovskoye | 1125 | 1207.6 | 1052.6 | 1052.6 | 1052.6 | 1052.6 | 869.5 | 731.2 |
| 82 | Tomicus minor | Pirovskoye | 43 | 72.6 | 72.6 | 48 | 59.6 | 59.6 | 59.6 | 59.6 |
| 83 | Tomicus piniperda | Pirovskoye | 31 | 48 | 48 | 0 | 48 | 48 | 48 | 34.4 |
| 84 | Scolytus ratzeburgi | Pirovskoye | 97 | 97 | 97 | 97 | 97 | 97 | 97 | 0 |
| 85 | Polygraphus proximus | Pirovskoye | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 3 |
| 86 | Xylechinus pilosus | Pirovskoye | 0 | 0 | 0 | 59.6 | 0 | 0 | 0 | 0 |
| 87 | Monochamus urussovi | Pirovskoye | 0 | 5570.2 | 5532.7 | 5532.7 | 5532.7 | 5532.7 | 5343.2 | 3442 |
| 88 | Xylechinus pilosus | Sayano-Shushenskoye | 1 | 1 | 1 | 53.9 | 0 | 0 | 0 | 0 |
| 89 | Dicerca aenea | Sayanskoye | 16 | 16.3 | 0 | 0 | 0 | 0 | 0 | 0 |
| 90 | Tomicus minor | Sayanskoye | 107 | 82.4 | 53.9 | 0 | 53.9 | 53.9 | 53.9 | 53.9 |
| 91 | Melanophila guttulata | Sayanskoye | 0 | 0 | 16.3 | 16.3 | 16.3 | 16.3 | 16.3 | 16.3 |
| 92 | Monochamus urussovi | Sayanskoye | 0 | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| 93 | Monochamus urussovi | Severo-Eniseyskoye | 0 | 330.5 | 330.5 | 309.2 | 25.9 | 25.9 | 25.9 | 5.9 |
| 94 | Monochamus urussovi | Sayano-Shushenskoye | 0 | 956 | 956.1 | 956.1 | 0 | 0 | 956.1 | 956.1 |
| 95 | Monochamus sutor | Tayozhinskoye | 73 | 68.9 | 24.9 | 24.9 | 20 | 20 | 20 | 20 |
| 96 | Polygraphus proximus | Tayozhinskoye | 0 | 0 | 0 | 0 | 0 | 0 | 275 | 355 |
| 97 | Monochamus urussovi | Tayozhinskoye | 0 | 2252.7 | 2135.2 | 1953.9 | 1310.4 | 1289.7 | 1281.4 | 1281.4 |
| 98 | Ips subelongatus | Teryanskoye | 0 | 0 | 0 | 0 | 0 | 0 | 93.2 | 93.2 |
| 99 | Tomicus piniperda | Teryanskoye | 0 | 0 | 0 | 0 | 0 | 0 | 70 | 70 |
| 100 | Monochamus urussovi | Teryanskoye | 0 | 759 | 759 | 759 | 759 | 759 | 654 | 654 |
| 101 | Ips subelongatus | Tungusso-Chunskoye | 48 | 30.7 | 30.7 | 30.7 | 30.7 | 0 | 30.7 | 30.7 |
| 102 | Ips sexdentatus | Tungusso-Chunskoye | 74 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 103 | Tomicus minor | Tungusso-Chunskoye | 0 | 0 | 0 | 27.1 | 0 | 0 | 0 | 0 |
| 104 | Tomicus piniperda | Tungusso-Chunskoye | 0 | 28.6 | 27.1 | 0 | 27.1 | 27.1 | 27.1 | 27.1 |
| 105 | Monochamus urussovi | Tungusso-Chunskoye | 0 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| 106 | Monochamus sutor | Tyuhtetskoye | 4 | 4 | 4 | 4 | 4 | 4 | 4 | 4 |
| 107 | Polygraphus proximus | Tyuhtetskoye | 0 | 0 | 0 | 0 | 0 | 38.3 | 306.3 | 278 |
| 108 | Monochamus urussovi | Tyuhtetskoye | 0 | 173 | 173 | 173 | 173 | 173 | 172.5 | 172.5 |
| 109 | Ips subelongatus | Uzhurskoye | 7 | 7 | 7 | 7 | 7 | 7 | 7 | 7 |
| 110 | Monochamus urussovi | Uzhurskoye | 0 | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| 111 | Melanophila guttulata | Usinskoye | 0 | 0 | 0 | 0 | 0 | 91 | 91 | 91 |
| 112 | Ips sexdentatus | Usinskoye | 0 | 0 | 0 | 0 | 0 | 209 | 209 | 209 |
| 113 | Monochamus urussovi | Usinskoye | 0 | 0 | 0 | 0 | 0 | 1499 | 1499 | 1499 |
| 114 | Monochamus urussovi | Usolskoye | 0 | 1666 | 1666 | 39 | 39 | 39 | 39 | 39 |
| 115 | Tomicus minor | Uyarskoye | 14 | 14 | 14 | 0 | 0 | 0 | 0 | 0 |
| 116 | Monochamus urussovi | Uyarskoye | 0 | 346.5 | 346.5 | 0 | 0 | 0 | 0 | 0 |
| 117 | Tomicus minor | Khrebtovskoye | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 118 | Tomicus piniperda | Khrebtovskoye | 0 | 84 | 84 | 84 | 84 | 84 | 84 | 84 |
| 119 | Monochamus urussovi | Khrebtovskoye | 0 | 768 | 768 | 768 | 717 | 717 | 717 | 717 |
| 120 | Tomicus minor | Chunskoye | 291 | 281 | 281 | 0 | 0 | 0 | 0 | 0 |
| 1121 | Monochamus sutor | Chunskoye | 3 | 1.5 | 1.5 | 1.5 | 1.5 | 21.5 | 21.5 | 21.5 |
| 1122 | Scolytus ratzeburgi | Chunskoye | 61 | 0 | 0 | 0 | 0 | 4 | 4 | 4 |
| 1123 | Dendroctonus micans | Chunskoye | 0 | 0 | 0 | 0 | 0 | 0 | 31 | 31 |
| 1124 | Monochamus urussovi | Chunskoye | 0 | 1552 | 0 | 0 | 956.1 | 956.1 | 17 | 17 |
Methods
In any community consisting of a large number of species, it is unlikely that any environmental factor affects only one species in the entire community. The effect of an environmental factor and the simultaneous reaction of several species to it may indicate the presence of an interaction between species, about possible connectivity into a complex within a community, about the presence of conjugation of population dynamics. This correlation is revealed as a correlation between the quantitative characteristics of species populations. There are two main types of contingency: the temporary conjugation of several species in one habitat and spatial conjugation of population dynamics of one species in different habitats. The temporal correlation is shown in the fact that the dynamics of the number of different species of the complex can occur synchronously in the same habitat, such as the rise in the number of Bupalus piniaria and the complex of phyllophages related to this species in the Krasnoturansky Bor in the Krasnoyarsk territory (Пальникова и др., 2014). The spatial conjugacy of a particular species can be characterized, for example, by the number of forest areas where outbreaks of its mass reproduction are observed at a given time.
When studying the spatial conjugacy of a certain species k in N = 62 habitats (forestries) during M = 8 years, the matrix A(k) = || aijk || dimension (N x M) was used, in which cell (i, j) presents data on the area of damage to the stands by species k in the i-th forestry in the j-th year. Such matrices were constructed for each of the P = 15 registered xylophage species.
When studying the temporal conjugacy of P species in habitat m, the matrix B(m)=||bijm|| of dimension (P x M) was used, in which data on the indicator of damage to plantings in this habitat by species i in year j are presented in cell (i, j). Such matrices were constructed for each of N = 62 forestries.
15 matrices A(k) were used to assess the spatial conjugation of population dynamics of an individual insect species in different habitats (forest areas). The values of correlation coefficients between rows of A(k) matrices for each of the 15 xylophage species studied were used for spatial conjugation calculations. As a result of such calculations, correlation matrices of spatial conjugacy dimension (N x N) were obtained for all 15 species of xylophages, in which a separate cell (i, j) characterized the spatial conjugacy development of outbreaks of mass reproduction of a separate xylophages species during 2007-2014 in forest areas i and j. If the absolute value of the correlation coefficient in the cell was significant according to standard statistical criteria, this indicated spatial correlations (in the case of negative values of the correlation coefficient – anticonjugation) of the development of foci of individual species in the two forest areas i and j. A positive value of the correlation coefficient in the cell of the correlation matrix indicated that with the emergence of the focus of mass reproduction of individual species within the forest area a similar lesion appears in another forest. If the correlation coefficient is negative, the appearance of a focus in one forest area was associated with the absence of a focus of the studied species in another forest area.
62 correlation matrices B(m) were used to estimate the time conjugacy of mass reproduction of different xylophage species in the same forest area. The values of correlation coefficients between rows of B(m) matrices were used for calculations. If the correlation coefficient was positive, it indicated that the presence of one species of the xylophage complex was a factor that influenced the appearance of another species, i.e. the presence of a temporary conjugation in the population dynamics between different xylophage species in the same habitat (forestry). If the correlation coefficient was negative, it indicated that the presence of one species in the focus did not affect the appearance of another species.
The statistical package Statistica 6.0 was used to calculate correlation matrices. The statistical significance of each correlation coefficient in the correlation matrices was estimated at the level of p = 0.90. If a separate correlation coefficient in the correlation matrix turned out to be insignificant at the selected level p, then it was concluded that there was no spatial synchronism of damage to plantations by pests of the same species in different habitats or a temporary synchronization of damage to plantations by pests of several species in one habitat.
Results
Time synchronization characterizes a situation when in a single territory outbreaks of several species are observed. For xylophages, this effect may be associated with changes in the state of forage woody plants on the territory. Different forestries in the Krasnoyarsk territory differ in the number of registered centers of mass reproduction of xylophages. The "record holder" for the number of foci of different species of xylophages is the Gremuchinsky forestry, where during 2007-2014 there were foci of mass reproduction of 11 species of xylophages. Also, a large number of foci of different species of stem pests were observed in the territories of the Kondinsky, Pirovsky, Mansky and Nevonsky forestries. In 11 forestries the centers of mass reproduction of only one species was observed; in 16 forestries there were no outbreaks of mass reproduction of xylophages in 2007-2014.
The distribution of forestries by the number of centers of mass reproduction of various species of xylophages is shown in Fig. 1. In almost two-thirds (65.6 %) of the total number of forestries (61), the number of xylophage enters does not exceed 2.

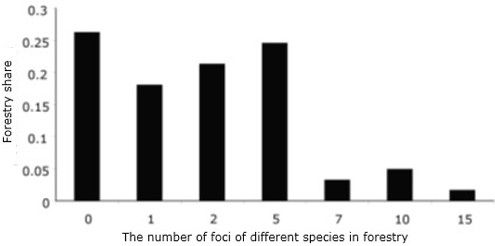
Fig. 1. Distribution of forest areas in Krasnoyarsk region by the number of outbreaks of various xylophages species
Distribution of centers of mass reproduction of xylophages in the territory of The Krasnoyarsk region depends on the natural and climatic conditions in which the forests are located. In table. 2 the characteristics of the occurrence of stem insect foci depending on the forest-plant area in which the forestry is located are given (Государственный доклад..., 2018).
Table 2. Occurrence of outbreaks of various species of xylophages depending on the forest-plant zone in which the forestry is situated
| Forest-plant zone | Total number of forestries | Number of forestries with registered outbreaks of mass breeding | The share of forestries with centers of mass reproduction | The average number of recorded outbreaks of breeding on a forestry |
| Taiga zone | 17 | 12 | 0.71 | 4.75 |
| Forest-steppe zone | 36 | 28 | 0.78 | 2.79 |
| South Siberian mountain zone | 9 | 5 | 0.55 | 2.00 |
The share of forest areas with centers of mass reproduction of stem pests for the taiga zone is 71 %; for the forest-steppe zone - 78 %, for the South Siberian mountain zone – 55 %. In all three forest zones, the average number of registered xylophage mass breeding centers is at least 2. For the taiga zone it is 4.75, for the forest-steppe zone – 2.79, for the South Siberian mountain zone – 2. Different types of xylophage insects differ greatly in the area of territories where their mass breeding centers appeared and operated (table. 3).
Table 3. Maximum annual area (ha) of xylophage foci in the territory of Krasnoyarsk
| Species rank | Xylophagous insect species | Maximum annual area of outbreaks, ha |
| 1 | Monochamus urussovi | 153085.0 |
| 2 | Polygraphus proximus | 8437.6 |
| 3 | Monochamus galloprovincialis | 7050.3 |
| 4 | Ips sexdentatus | 3250.7 |
| 5 | Tomicus piniperda | 2031.5 |
| 6 | Ips typographus | 1223.8 |
| 7 | Tomicus minor | 928.0 |
| 8 | Melanophila guttulata | 809.1 |
| 9 | Ips subelongatus | 314.0 |
| 10 | Scolytus ratzeburgi | 218.0 |
| 11 | Dicerca aenea | 184.0 |
| 12 | Monochamus sutor | 87.0 |
| 13 | Xylechinus pilosus | 55.0 |
| 14 | Saperda carcharias | 35.0 |
| 15 | Dendroctonus micans | 31.0 |
From the data of the table. 3 it can be seen that the size of the centers of mass reproduction of different species of xylophages differed significantly. Thus, the maximum annual area of foci of mass reproduction of Monochamus urussovi (153085 ha) is almost 5000 (!) times more than the maximum annual area of Dendroctonus micans foci (31 ha).
As an example of calculating the time conjugation of the occurrence of foci of mass reproduction of various xylophage species in one forestry, in the table 4 it is shown the area of damaged forest plantations (ha) on territory of Gremuchinsky forestry (data are selected from table 1). In the course of forest pathological surveys of forest plantations during 2007-2014, 9 xylophage species were observed in the Gremuchinsky forestry. The mass reproduction of Xylechinus pilosus was noted only once, and the area of the plantations damaged by it was just over 15 hectares. Saperda carcharias propagated in mass during 2012-2014, damaging stands on a small area. Every year in the plantations of the Gremuchinsky forestry there are foci of Ips sexdentatus, Tomicus minor, and Monochamus urussovi (since 2008). Complex centers of mass reproduction of xylophages are formed as a result of the increase in the number of 5-6 species of insects at the same time. Since 2012, 7 xylophage species have been registered simultaneously in outbreak foci (see table. 4).
Table 4. Areas (ha) of outbreak foci of various species of insects-xylophages on the territory of the Gremuchinsky forestry
| Xylophagous insect species | The area of foci (ha) of stem insects by years | |||||||
| 2007 | 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |
| Dicerca aenea | 45 | 45 | 0 | 0 | 0 | 0 | 0 | 0 |
| Ips subelongatus | 41 | 41 | 41 | 5.4 | 5.4 | 188.1 | 78.4 | 30.2 |
| Ips sexdentatus | 423 | 213.6 | 213.6 | 213.6 | 213.6 | 213.6 | 213.6 | 213.6 |
| Tomicus minor | 298 | 297.8 | 297.8 | 23.1 | 15.2 | 15.2 | 15.2 | 80 |
| Tomicus piniperda | 39 | 25.1 | 25.1 | 0 | 23.1 | 259 | 121.3 | 48.6 |
| Melanophila guttulata | 0 | 0 | 45 | 45 | 45 | 45 | 45 | 96.3 |
| Saperda carcharias | 0 | 0 | 0 | 0 | 0 | 35 | 35 | 12.8 |
| Xylechinus pilosus | 0 | 0 | 0 | 15.2 | 0 | 0 | 0 | 0 |
| Monochamus urussovi | 0 | 3287.4 | 3279.6 | 3241.2 | 3241.2 | 3241.2 | 3203.2 | 3203.2 |
By the data from the table. 4, according to the above calculation method, the correlation matrix of the time conjugation of foci of different xylophage species in the territory of this forestry was calculated (table. 5).
Table 5. Correlation matrix of the temporary conjugation of xylophage outbreaks in the Gremyachinsky forestry
| № of species | Xylophagous insect species * | ||||||||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
| 1 | 1.000 | -0.134 | 0.655** | 0.737* | -0.258 | -0.814* | -0.403 | -0.218 | -0.642 |
| 2 | 1.000 | -0.088 | -0.220 | 0.976* | -0.005 | 0.804* | -0.331 | 0.085 | |
| 3 | 1.000 | 0.483 | -0.136 | -0.533 | -0.264 | -0.143 | -1.000* | ||
| 4 | 1.000 | -0.405 | -0.590 | -0.559 | -0.308 | -0.464 | |||
| 5 | 1.000 | 0.123 | 0.881* | -0.321 | 0.129 | ||||
| 6 | 1.000 | 0.313 | 0.064 | 0.516 | |||||
| 7 | 1.000 | -0.264 | 0.249 | ||||||
| 8 | 1.000 | 0.142 | |||||||
| 9 | 1.000 | ||||||||
Note. * – species: 1 – Dicerca aenea, 2 – Ips subelongatus, 3 – Ips sexdentatus, 4 – Tomicus minor, 5 – Dendroctonus micans, 6 – Melanophila guttulata, 7 – Saperda carcharias, 8 – Xylechinus pilosus, 9 – Monochamus urussovi. ** – correlations are significant at the level of p = 0.90.
As it is known, for species i and j, the correlation coefficient r (i, j) = r (j, i), so the correlation matrix is symmetric with respect to the main diagonal, and data below the main diagonal can be omitted. As shown in table 5, there may be a positive conjugacy of the occurrence of foci of two species in the same forestry (the correlation coefficient r > 0 and is significant at the level of p = 0.90) and a negative conjugation of foci of two species in the same forestry (r < 0 and is significant at the level of p = 0.90). In the first case, if there is a focus of one species in the forestry, for the most part a focus of a positively conjugate species is also found. Thus, the centers of mass reproduction of the Ips subelongatus (species 2) and the Tomicus piniperda (species 5) are positively associated. For these species, the correlation coefficient r (2, 5) = +0.976. The centers of mass reproduction of Ips subelongatus (species 2) and Saperda carcharias (species 7) are positively associated too. For these species, the correlation coefficient r (2, 7) = + 0.804.
In a case of negative conjugacy, if there is a focus of one species, the foci of another species do not occur. This is the case with the foci of Ips sexdentatus (species 3) and the Monochamus urussovi (species 9). For these species, the correlation coefficient r (3, 9) = - 1.0.
On the basis of calculations of time conjugation correlation matrices of foci of mass reproduction of the xylophage insect complex in various forestries of the Krasnoyarsk territory, a positive conjugation in the dynamics of a number of xylophage species was revealed (table 6).
M. galloprovincialis (see table 6) is the species that is associated with the largest number of foci of other species-xylophages of this complex. This species forms conjugate foci with 10 other species, of which the foci of Monochamus galloprovincialis and Scolytus ratzeburgi are most often interconnected. Monochamus galloprovincialis damages all coniferous trees, but most strongly – the scotch pine. When passing additional nutrition in the crowns of trees, beetles gnaw the bark of thin branches, which significantly weaken the trees during mass reproduction. In addition to coniferous trees, beetles can damage branches and hardwoods such as birch and aspen. Scolytus ratzeburgi is widespread and damages, as a rule, old and weakened birch trees. Since these species do not compete for food resources, the conjugacy of their foci, according to Moran (1953), may be related to the common requirements for climatic conditions.
Table 6. Positive temporary conjugation of foci of certain types of xylophages
| Xylophagous insect species | The number of conjugated species | The most common interconnected species |
| Monochamus galloprovincialis | 10 | Scolytus ratzeburgi |
| Tomicus piniperda | 8 | Tomicus minor |
| Ips sexdentatus | 8 | Monochamus galloprovincialis |
| Monochamus urussovi | 8 | Monochamus galloprovincialis |
| Scolytus ratzeburgi | 7 | Monochamus urussovi |
| Melanophila guttulata | 6 | Dicerca aenea |
| Polygraphus proximus | 6 | Monochamus urussovi |
| Dicerca aenea | 5 | Melanophila guttulata |
| Ips subelongatus | 5 | Monochamus galloprovincialis |
| Ips typographus | 4 | |
| Tomicus minor | 4 | Monochamus galloprovincialis |
| Dendroctonus micans | 3 | |
| Xylechinus pilosus | 3 | Tomicus piniperda |
| Saperda carcharias | 2 | |
| Monochamus sutor | 2 |
Quite a large conjugation with foci of other species is characteristic for Tomicus piniperda, Ips sexdentatus and Monochamus urussovi. The number of related stem insect species with these three species is 8. At the same time, the most frequently paired species are Tomicus minor and Monochamus galloprovincialis.
Tomicus minor as Tomicus piniperda are found in the area of pine everywhere. The beetles feed on weakened trees. Their settlement area – the peaks, large branches, and the central part of tree trunks of various ages. In addition, the Tomicus by "haircut" weakens healthy, not yet populated by it pine trees, thereby preparing the base for further setting.
Tomicus piniperda attack weakened pines, often form foci on burning areas, in the foci of the pine fungus, in conditions of development pressure. These species belong to the spring phenological complex of stem pests.
Monochamus urussovi develop in all coniferous species. Beetles additionally feed on needles and bast on the shoots and branches of living trees. Monochamus urussovi in the forests of Siberia and the far East, breeds in huge number in Siberian silkworm foci, in burning areas, and in areas of large logging operations. All Monochamus inhabit both standing and fallen trees (Лесная энтомология, 2010). These species can act as competitors, but still form conjugate (complex) foci. This is due to the fact that the Tomicus piniperda, Tomicus minor, and Monochamus are capable of additional feeding in the imaginal phase. Additional feeding is carried out by beetles on shoots and branches viable trees, weakening them and expanding their food base. In addition, the joint mastering of woody plants by different species of xylophages allows to overcome the resistance of the physiological tree systems (Исаев, Гирс, 1975).
The spatial conjugacy of outbreaks of mass reproduction of a particular species can be characterized by the number of forest areas where outbreaks of its mass reproduction are observed. So, the centers of mass reproduction of Monochamus urussovi met in 36 forestries, Monochamus galloprovincialis – in 20, Tomicus minor and Polygraphus proximus – in 13 forestries. Among the most common species in the territory of the region are the Tomicus piniperda and Ips typographus (in 10 forest districts). Saperda carcharias with a 35-hectare outbreak was recorded on the territory of one forestry (see table 1). The reasons for spatial conjugation can be the synchronization of population dynamics and the state of forage plants, the similarity of the reaction of populations to weather changes in different habitats.
The risk of outbreaks of mass reproduction of a particular xylophagus species in the territory in the region can be characterized using the following indicators: the share of forest areas where outbreaks of this species occur, indicators of the conjugacy of outbreaks of this species in different forestries, the area of outbreaks of mass reproduction of this species in relation to the total area of forest plantations. The higher these values, the more risk of outbreaks of this species of xylophage.
As an illustration, we present an assessment of the risk of an outbreak of mass reproduction of Monochamus urussovi in forest areas in the Krasnoyarsk territory during 2007-2014.
Having data on the area of outbreaks of Monochamus urussovi in the forestry space of the Krasnoyarsk territory and time (2008-2014) (table 7), one can calculate the correlation matrix of spatial conjugation of Monochamus urussovi outbreaks in various forestries in the Krasnoyarsk territory. In table 8 a fragment of this correlation matrix is given (it is difficult to give the entire correlation matrix due to its large size).
The analysis of table 8 allows to identify areas with synchronous centers of mass reproduction of Monochamus urussovi. For example, the dynamics of development of the outbreaks of Monochamus urussovi matches in Gremuchinsky, Eniseysky and Usolsky forestries (correlation coefficients more than 0.78), whereas temporal dynamics of the development of outbreak of this species anticorrelated in Achinsky and Kizirsky forestry. Thus, the share of forestries where outbreak of this species is observed is equal to 0.581. For the entire complex of xylophages considered, information on the area of outbreaks and the percentage of forestries where outbreaks occur is given in table 9.
The risks of developing outbreaks of mass reproduction of various xylophage species in the territories of different forestries can be represented graphically in Fig. 2, where on the abscissa axis in this graph is the share q of forestries in which outbreaks of this species are observed, and on the ordinate axis is the logarithm of the share S0 of the area of foci of this species from the total territory of the whole forests region. A point on the plane characterizes a separate species. For example, there are points that characterize the Monochamus urussovi (point 1), Monochamus galloprovincialis (point 2), and Ips sexdentatus (point 3).
Points in the lower left corner of the plane {q, ln S0} characterize species with a low level of impact on the forest (the percentage of forest areas where these xylophage species occur is small, and the areas of foci are small). Thereto group belong the majority of species of studied entomocomplexes: Dendroctonus micans, Saperda carcharias, Xylechinus pilosus, Monochamus sutor, Dicerca aenea, etc. Points in the upper right corner of the plane {q, ln S0} characterize the xylophagous species with a strong impact on the forest (the centers of mass reproduction of these species occur in most forestries on the territory of Krasnoyarsk region and the area of centers are large). Species from this group include Monochamus urussovi, Monochamus galloprovincialis, Tomicus minor, Polygraphus proximus. In the upper-left corner of the plane {q, ln S0} on Fig. 2 there should be points that characterize locally impacting species (occurring in a small number of forestries, but the areas of centers in these forestries are large). In the lower right corner of the plane {q, ln S0} there should be points that characterize diffusely affecting species with centers in a large number of forestries, but with a small area. However, as can be seen in Fig. 2, locally and diffusely affecting xylophage species were not found in the territory of forestries of the Krasnoyarsk region during the research period.
Discussion
The problem of the occurrence of foci and the frequency of outbreaks of mass reproduction of dendrophilic insects for many decades occupies a leading place in ecological studies in the world. In the mid-twenties of the twentieth century, ecologists put forward theoretical ideas about the periodicity of mass reproduction, their interaction with the cycles of solar activity, climate, and natural enemies (entomophages). The main factor in the dynamics of the number of stem insects is the quantity and quality of food. Weather and other ecological factors have an indirect effect on population dynamics through the state of fodder plants.
In domestic and foreign ecological literature for a long time the question is raised about the relationship of insect population cycles with climatic factors Domestic and foreign ecological literature long ago has raised the question about the relationship of insect population cycles with climate factors. It is known (Берриман, 1990) that cycles of different populations of the same species separated by large distances can take place synchronously with each other. The mechanisms that ensure a synchronous increase in the number of insect populations over a wide area are not yet known in details. It is assumed that the cause of synchronization of outbreaks of mass reproduction of insects may be some external factor, the impact of which leads to the simultaneous development of local outbreaks. In particular, such synchronizing factors can be changes in the Sun activity (Чижевский, 1973), summer droughts over a wide area (Кондаков, 1974, 2002). Since the rhythm of the solar activity determines the dynamics of the impact of solar radiation simultaneously on the entire planet, and the number of outbreaks of number of insect species are not synchronized in time and space, it is assumed that the synchronizing factor is a combination of the rhythm of solar activity and local planetary rhythms (Moran, 1953). According to different authors, the reason for the conjugation of population dynamics of one species in different habitats may be the effect of P. Moran, associated with the uniformity of climatic conditions over a large area and the similarity of the reaction of populations to weather changes in different habitats (Максимов, 1989; Bjornstad, Bascompte, 2001; Пальникова и др., 2014). It is shown (Liebhold, Kamata, 2000; Liebhold et al., 2004), that the degree of conjugation of population dynamics of one species in different habitats monotonously decreases with increasing distance between these habitats. If the level of conjugacy of population dynamics does not decrease with increasing distance between habitats, and the distance between them significantly exceeds the radius of individual movement of animals of the studied species, then we should talk about its global spatial coherence associated with the reaction of populations to the influence of a powerful modifying factor.
Table 7. Areas of outbreak foci for Monochamus urussovi Fisch. on Krasnoyarsk Region territory for 2008–2014
| Forestry | The area of foci of Monochamus urussovi Fisch.(ha) by years | ||||||
| 2008 | 2009 | 2010 | 2011 | 2012 | 2013 | 2014 | |
| Achinskoye | 13.7 | 13 | 13 | 13 | 13 | 13 | 13 |
| Balakhtinskoye | 41.5 | 35.3 | 33.2 | 0 | 0 | 0 | 0 |
| Bogotolskoye | 373.8 | 350.8 | 350.8 | 350.8 | 350.8 | 25 | 25 |
| В.-Manskoye | 659 | 659 | 609 | 609 | 609 | 609 | 609 |
| Gremuchinskoye | 3287.4 | 3279.6 | 3241.2 | 3241.2 | 3241.2 | 3203.2 | 3203.2 |
| Mininskoye | 8.1 | 8.1 | 8.1 | 0 | 0 | 0 | 0 |
| Dzerzhinskoye | 0 | 0 | 0 | 0 | 2 | 2 | 2 |
| D.-Mostovskoye | 95.3 | 95.3 | 205.3 | 110 | 110 | 110 | 110 |
| Eniseyskoye | 10081 | 10144 | 1711 | 2227 | 2227 | 831 | 831 |
| Ermakovskoye | 3280.4 | 3280.4 | 3280.4 | 3280.4 | 3280.4 | 3140 | 3140 |
| Irbeyskoye | 35508 | 35508 | 35508 | 35508 | 27250 | 26520 | 0 |
| Kazachinskoye | 7750 | 150 | 150 | 0 | 0 | 0 | 0 |
| Karatuzskoye | 4065 | 4065 | 3434 | 3434 | 3567 | 1732 | 1732 |
| Kodinskoye | 2391 | 2391 | 2391 | 2391 | 2391 | 2293 | 2578.9 |
| Kozulskoye | 3.9 | 3.9 | 3.9 | 3.9 | 3.9 | 0 | 0 |
| Krasnoyarskoye | 89.5 | 84 | 84 | 0 | 0 | 0 | 0 |
| Kizirskoye | 798 | 2961.1 | 2669.3 | 2650.9 | 2496.2 | 2496.2 | 2048.2 |
| Maganskoye | 83 | 83 | 82.5 | 82.5 | 82.5 | 82.5 | 82.5 |
| Manzenskoye | 67.1 | 48.5 | 22 | 22 | 22 | 22 | 22 |
| Manskoye | 574.2 | 571.4 | 598.1 | 583.2 | 583.2 | 583.2 | 583.2 |
| Motyginskoye | 42143.6 | 40644.6 | 40361.6 | 40361.6 | 40361.6 | 35780.6 | 15342 |
| Nevonskoye | 104.9 | 104.9 | 104.9 | 104.9 | 104.9 | 137.9 | 137.9 |
| Pirovskoye | 5570.2 | 5532.7 | 5532.7 | 5532.7 | 5532.7 | 5343.2 | 3442 |
| S.-Shushenskoye | 956 | 956.1 | 956.1 | 0 | 0 | 956.1 | 956.1 |
| Sayanskoye | 1.2 | 0 | 0 | 0 | 0 | 0 | 0 |
| С.-Eniseyskoye | 330.5 | 330.5 | 309.2 | 25.9 | 25.9 | 25.9 | 5.9 |
| Tayozhinskoye | 2252.7 | 2135.2 | 1953.9 | 1310.4 | 1289.7 | 1281.4 | 1281.4 |
| Teryanskoye | 759 | 759 | 759 | 759 | 759 | 654 | 654 |
| Т.-Chunskoye | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Tyuhtetskoye | 173 | 173 | 173 | 173 | 173 | 172.5 | 172.5 |
| Uzhurskoye | 35 | 35 | 35 | 35 | 35 | 35 | 35 |
| Usolskoye | 1666 | 1666 | 39 | 39 | 39 | 39 | 39 |
| Uyarskoye | 346.5 | 346.5 | 0 | 0 | 0 | 0 | 0 |
| Khrebtovskoye | 768 | 768 | 768 | 717 | 717 | 717 | 717 |
| Chunskoye | 1552 | 0 | 0 | 956.1 | 956.1 | 17 | 17 |
| Usinskoye | 0 | 0 | 0 | 0 | 1499 | 1499 | 1499 |
Table 8. A fragment of the correlation matrix of spatial contiguity of the foci of the Monochamus urussovi Fisch in various forestries in the Krasnoyarsk Territory
| The number of forestry | The number of forestry * | |||||||
| 1 | 5 | 7 | 9 | 16 | 17 | 20 | 32 | |
| 1 | 1.00 | 0.60** | -0.35 | 0.64* | 0.51 | -0.92* | -0.42 | 0.65* |
| 5 | 1.00 | -0.76* | 0.91* | 0.78* | -0.30 | -0.51 | 0.85* | |
| 7 | 1.00 | -0.60* | -0.75* | 0.06 | 0.09 | -0.55 | ||
| 9 | 1.00 | 0.75* | -0.37 | -0.75* | 0.99* | |||
| 16 | 1.00 | -0.25 | -0.14 | 0.74* | ||||
| 17 | 1.00 | 0.31 | -0.40 | |||||
| 20 | 1.00 | -0.76* | ||||||
| 32 | 1.00 | |||||||
Note. * – forestries: 1 – Achinskoye, 5 – Gremuchinskoye, 7 – Dzerzhinskoye, 9 – Yeniseiskoye, 16 – Krasnoyarskoye, 17 – Kizirskoye, 20 – Manskoye, 32 – Usolskoye. ** – the correlation coefficient is significant at p = 0.90.
Table 9. Occurrence of outbreaks foci of certain xylophages species in forest areas of Krasnoyarsk Region during 2007–2014
| Species of insects- xylophages | Maximal annual area of foci, ha | Number of forestries with foci of this species | Percentage of forestries with foci of this species | The ratio of the areas of foci to the areas of forest plantations |
| Monochamus urussovi | 153085 | 36 | 0.581 | 0.00156 |
| Polygraphus proximus | 8437.6 | 13 | 0.210 | 8.61*10-5 |
| Monochamus galloprovincialis | 7050.3 | 20 | 0.323 | 7.19488*10-5 |
| Ips sexdentatus | 3250.7 | 2 | 0.032 | 3.31736*10-5 |
| Tomicus minor | 2031.5 | 10 | 0.161 | 2.07316*10-5 |
| Ips typographus | 1223.8 | 10 | 0.161 | 1.2489*10-5 |
| Tomicus minor | 928 | 13 | 0.210 | 9.47031*10-6 |
| Melanophila guttulata | 809.1 | 6 | 0.097 | 8.25692*10-6 |
| Ips subelongatus | 314 | 9 | 0.145 | 3.20439*10-6 |
| Scolytus ratzeburgi | 218 | 7 | 0.113 | 2.22471*10-6 |
| Dicerca aenea | 184 | 4 | 0.065 | 1.87773*10-6 |
| Monochamus sutor | 87 | 6 | 0.097 | 8.87841*10-7 |
| Xylechinus pilosus | 55 | 5 | 0.081 | 5.61279*10-7 |
| Saperda carcharias | 35 | 1 | 0.016 | 3.57177*10-7 |
| Dendroctonus micans | 31 | 2 | 0.032 | 3.16357*10-7 |
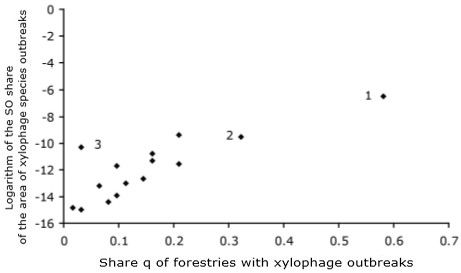
Fig. 2. The risks of outbreaks of various xylophages species
The dynamics of the foci distribution, the synchronicity of their formation in space and the degree of synchronicity of the foci formation of different species in the same habitat are of practical importance. However, this requires materials on the inventory population outbreaks of dendrophilic species in forestries, performed by specialists. The use of spatial correlation matrices for a particular species allows, after the detection of foci of mass reproduction of this species of xylophage in a single forestry to assess the risks of outbreaks of this species in other forestries. The use of the correlation matrix of the temporal conjugation of the dynamics of several species in a separate forestry makes it possible to assess the risks of developing outbreaks of other xylophagous species in this forestry when a focus of mass reproduction of one xylophage species is detected.
Conclusions
1. The most dangerous species of xylophagous insects at risk of impact to forest stands in the Krasnoyarsk region are: Monochamus urussovi Fisch., Polygraphus proximus Blandf., Monochamus galloprovincialis Ol., Tomicus piniperda L.
2. To assess the risk of developing of conjugated foci of a number of species in a single forestry, one can use correlation matrices of time conjugation of xylophage populations. For example, for Gremuchinskoye forestry it is a high risk of concurrent development (temporary conjugation) of several species of xylophages: Ips subelongatus and Tomicus piniperda, Ips subelongatus and Saperda carcharias, Tomicus piniperda and Saperda carcharias, Dicerca aenea and Tomicus minor, Dicerca aenea and Ips sexdentatus.
3. To assess the risk of spatial conjugation of mass reproduction of a particular species of xylophage in different forestries, one can use correlation matrices of spatial conjugation of foci of this species. The highest degree of spatial conjugation of foci is typical for Monochamus urussovi, Monochamus galloprovincialis, Polygraphus proximus. This indicates the spatial distribution of these species, one should expect the simultaneous appearance of breeding centers of these species on the territory of different forestries.
References
Berriman A. Forest protection from insects pests. M.: VO Agropromizdat, 1990. 288 p.
Bjornstad O., Bascompte J. Synchrony and second order spatial correlation in host–parasitoid system, Journal of Animal Ecology. 2001. Vol. 70. P. 924–933.
Chizhevskiy A. L. Eath's echo of solar storms. M.: Mysl', 1973. 349 p.
Forest entomology: textbook for University students, E. G. Morozova, A. V. Selihovkin, P. P. Izhevskiy i dr.; pod red. E. G. Mozolevskoy. M.: Izdatel'skiy centr «Akademiya», 2010. 416 p.
Isaev A. S. Girs G. I. Tree and insects-xylofages interaction. Novosibirsk: Nauka, 1975. 348 p.
Kondakov Yu. P. Outbreaks of Siberian silkworm in the forests of Krasnoyarsk Region, Entomologicheskie issledovaniya v Sibiri. Krasnoyarsk: KF REO, 2002. Vyp. 2. P. 25–74.
Kondakov Yu. P. Regularities of outbreaks of Siberian silkworm. Novosibirsk: Nauka, 1974. P. 206–265.
Liebhold A., Kamata N. Are population cycles and spatial synchrony universal characteristics of forest insect population?, Population Ecology. 2000. Vol. 42. P. 205–209.
Liebhold A., Sork V., Peltonen M., Koenig W., Bjørnstad O. N., Westfall R., Elkinton J., Knops J. M. H. Within-population spatial synchrony in mast seeding of North American oaks, Oikos. 2004. Vol. 104. P. 156–164.
Maksimov A. Natural cycles: the causes of recurrence of ecological processes. L.: Nauka, 1989. 236 p.
Moran P. A. P. The statistical analysis of the Canadian lynx cycle. II. Synchronization and meteorology, Australian Journal of Zoology. 1953. Vol. 1. P. 291–298. DOI: 10.1071/zo9530291.
Pal'nikova E. N. Suhovol'skiy V. G. Tarasova O. V. Temporal and spatial coherence of population dynamics of forest insects phyllophages, Evraziatskiy entomologicheskiy zhurnal. 2014. No. 13 (3). P. 228–236.
State report " about the state and protection of the environment in Krasnoyarsk Region in 2017". Krasnoyarsk, 2018. P. 171–172. URL: http://mpr.krskstate.ru/dat/File/3/doklad%202017. pdf.





 © 2011 - 2026
© 2011 - 2026