Introduction
Bacteria of the genus Azotobacter belong to the free-living nitrogen fixers of the soil (rhizobacteria) and are able, like nodular bacteria of plants (rhizobia), with the help of a nitrogenase complex to fix molecular nitrogen of the air, turning it into an ammonium ion (Howard, Rees, 2006; Wani et al., 2013; Feoktistova et al., 2016). Bacteria of the genus Azotobacter inhabit ectorhizosphere (soil zone on the outside of the root) and rhizoplana (the surface of root system) of different species of nonlegurninous plants, using the exudates of the root system for feeding. In exchange, the plant receives nitrogen in the form of compounds available for assimilation; phosphorus nutrition of plants improves due to the dissolution of hard-to-reach soil phosphates in the process of vital activity of rhizobacteria. Phitohormones produced by rhizobacteria stimulate plant growth; Azotobacter bacteria inhibit the development of phytopathogenic fungi and bacteria (Feoktistova at al., 2016). The ability of various strains Azotobacter chroococcum Beijer to influence the seed germination and shoot development has been insufficiently studied (Kirchenko et al,2010). Although his issue is currently receiving considerable attention in connection with the search for effective strains of this genus in order to use them to increase the productivity of crops (Kirichenko, Kotz, 2011). There is information that some strains А. Сhroococcum are able to enter the symbiotic relationship with soft wheat (Triticum aestivum L.) (Kirichenko, 2016). However, the similar data for the strains А. сhroococcum of the soils in Nizhny Novgorod region are fragmentary, including for the strain No. 4 isolated by us from the soil of agricultural lands of Nizhny Novgorod region. It is known that bacteria of the genus Azotobacter form associations with pectinolytic and cellulose-destroying bacteria of the genus Bacillus by consuming the products of polymer decomposition by bacilli, supplying them with fixed nitrogen, which leads to the acceleration of polymer assimilation and stimulation of nitrogen fixation (Feoktistova et al., 2016). In this regard, when studying the ability of А. сhroococcum to directly influence the plant state, more objective data can be obtained under experimental conditions when cultivating plants on a nutrient solution. In this way it is possible to isolate the interaction in the system "plant-A. сhroococcum " in its pure form, i.e. without the participation of other species of soil bacteria.
In this regard, we first evaluated the ability of strain No. 4 A. chroococcum, isolated from the soil of agricultural lands of the Nizhny Novgorod region (Russia), to influence the germination of seeds and the state of wheat seedlings (Triticum aestivum L.) when different amounts of cells are added to the nutrient solution in the first day of the experiment in a wide range of values (from 109 CL/ml (A) to A/256).
Materials
Strain № 4 А. сhroococcum was isolated from the soil of arable land near the village Oranki (Bogorodsky district, Nizhegorodky region). А. сhroococcum was cultivated on liquid nutrient medium Eshby. In the experiment the seeds of winter wheat Moskovskaya 39 were used
Methods
For the study, 9 bacterial cell concentrations were selected from 109cells/ml (A) up to values several orders smaller (A/256) (the neighboring concentrations differed by 2 times), since the highest concentrations of A. chroococcum cells studied by us are used for inoculations of wheat seeds by other strains of this species (Kirichenko, 2016). Plants were grown for 8 days in a Knop nutrient solution with different contents of A. chroococcum cells (experimental group) or Knop solution (control group). In the dishes of experimental groups, the Knop solution with the addition of A. chroococcum was introduced only on the first day of the experiment. Then every day Knop solution was added to all dishes (control and experimental) without cells of nitrogen fixer. Wheat seeds, Knop solution and Petri dishes were not sterilized in order to determine whether this strain has an effect on the studied wheat indicators in non-sterile conditions. It is due to the fact that in practice, the treatment of seeds of agricultural crops with different strains of A. chroococcum is carried out before sowing in the soil, which eliminates the conditions of sterility. In each group, the plants were grown in 5 Petri dishes (50 seeds in each dish) on a substrate of filter paper moistened with a solution, at a 17-hour light period and a temperature of 17-22 °C.
In the first leaf of seven-day seedlings the intensity of lipid peroxidation (POL), the level of chlorophylls and carotenoids were determined as well as the raw biomass of the root system and shoot and seed germination. When studying biochemical parameters, there were 10 biological repetitions in each group (1 biological repetition is combined fragments of the first leaf of 5-6 different plants of this group; 2 biological repetitions were taken from each dish). The biomass of the root system and shoot was determined in 30 plants of each group (6 plants were taken from each dish; 6 plants x 5 dishes = 30). The intensity of POL was assessed by the content of TBA-active lipoperoxidation products, among which malon dialdehyde (MDA) is the most abundant (Kamyshnikov, 2002). The content of chlorophylls and carotenoids in the leaf was determined according to the generally accepted method, 80% acetone was used for extracting pigments (Shlyk, 1971).
The correspondence of the distribution in the samples of the studied quantitative features to the normal one was determined using the Shapiro-Wilk test (Statistica 10 program). Since in some samples the distribution was different from the normal one (p < 0.05), nonparametric Kruskal–Wallis and Newman–Kales criteria were used to test the null hypothesis (Biostatistics 4.03). A similar procedure was performed for a qualitative feature (seed germination) using the Chi-square criterion (Biostatistics 4.03) with the Bonferroni correction for multiple testing. The graphs show sample medians and their errors (quantitative characteristics), as well as shares and their errors (seed germination).
Results
All concentrations of the studied strain of A. chroococcum, with the exception of the smallest, resulted in decrease in germination of T. aestivum seeds by 14-30 % compared to the control (p < 0.05) (Fig. 1).
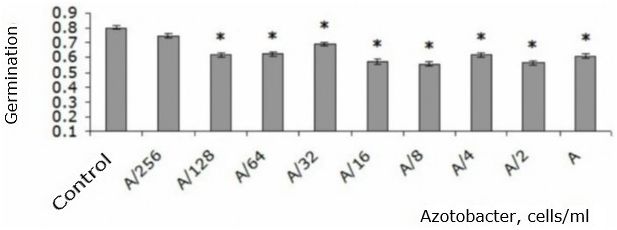
Fig. 1. T. aestivum seed germination when adding different amounts of A. chroococcum cells to nutrient solution in the first day of the experiment (share ± share error): * – indicates statistically significant differences compared to this indicator in the control group at p < 0.05; А – 109 c/ml
The statistically significant differences between the effects of different concentrations (p > 0.05) were not found.
Low concentrations of A. chroococcum (A/256-A/64) did not affect the mass of the root system (Fig. 2A). Concentrations of A/16-A/32 increased this indicator by 34-38 % relative to the control level. However, a further increase in the concentration to A/8 led to the disappearance of the effect. The highest concentrations of A. chroococcum A/4-A again increased the root mass of T. aestivum seedlings (by 50-68 % relative to control) (see Fig. 2A).
The dynamics of changes in the biomass of T. aestivum shoot with a decrease in the concentration of A. chroococcum was very similar to the change in this indicator in the root system, as evidenced by a strong positive correlation between these parameters (according to Spearman: r = 0.86; p < 0.05). However, the stimulating effect was less pronounced, so it was only recorded for concentrations of A/2 and A/4. They resulted in an increase in the shoot biomass by 21 and 30% compared to the control, respectively. The lower concentrations and the highest of the studied concentrations did not affect this indicator (Fig. 2B).
The intensity of lipid peroxidation in the leaves of wheat seedlings under the action of all studied concentrations of A. chroococcum did not differ from the control level (p > 0.05) (data in the figures are not presented). The content of chlorophylls and carotenoids in plants of all experimental groups corresponded to the control level (p > 0.05) (data are not presented in the figures).
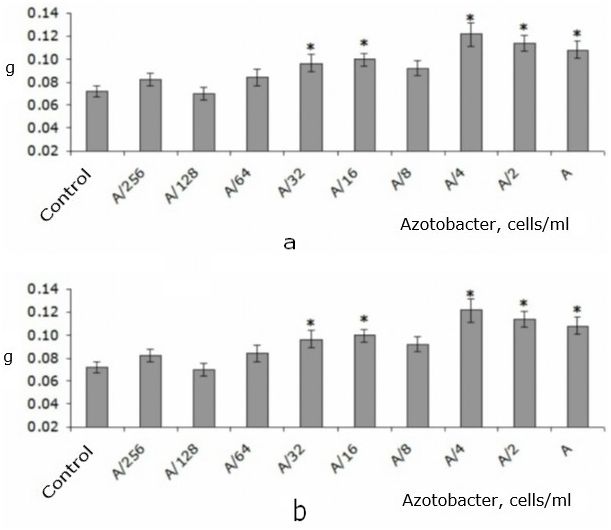
Fig. 2. Raw biomass of root system (a) and shoot (b) of T. aestivumwith adding of different amounts of A.chroococcum cells to nutrient solution in the first day of the experiment (Me ± SMe): * – indicates statistically significant differences compared to this indicator in the control group at p < 0.05; А – 109 c/ml
Discussion
Previously, some authors have shown that Azotobacter bacteria can stimulate the growth of the root system and plant shoots (Feoktistova et al., 2016). It is believed that rhizobacteria, including those of the genus Azotobacter, produce this effect by synthesizing phytohormones that stimulate growth (auxins, cytokinins, gibberellins) and improving the nitrogen and phosphorus nutrition of plants (Feoktistova et al., 2016). This mechanism may also underline the stimulating effect of bacteria on wheat growth that we found.
It is known that the dry seed contains a large amount of the phytohormone indolyl-3-acetic acid, which in high concentration together with the abscisic acid of the seeds inhibits germination (Nefedyev et al., 2013). Azotobacter bacteria are able to synthesize auxins, including indolyl-3-acetic acid (Feoktistova et al., 2016). Most likely, the inhibitory effect of nitrogen-fixing bacteria on the germination of wheat seeds is caused by the action of auxins produced by them.
It should be noted that the change in the biomass of the shoot and root system detected by us in T. aestivum seedlings when different concentrations of A. chroococcum cells were added to the nutrient solution was non-monotonic, since the highest of the studied concentrations did not affect the shoot biomass. Then this effect appeared in concentrations of A/2-A/4 and disappeared in lower numbers of bacterial cells. The biomass of the root system also underwent a non-monotonic change when the concentration of A. chroococcum cells decreased: concentrations of A-A/4 increased the biomass, A/8 did not affect, A/16-A/32 increased, and A/64-A/256 did not affect (see Fig. 2).
In recent years, it has been widely accepted that biosystems have a wide range of non-monotonic responses under the influence of various environmental factors (Calabrese, Blain, 2005). For example, we previously found that various chemical pollutants can often lead to non-monotonic changes in morphological, as well as physiological and biochemical parameters in different plant species (Erofeeva, 2014). Similar data are available for plant phytohormones, including auxins. It is shown that they can cause a multidirectional effect or not have it, as a result of which the "dose – effect" relationship is non-monotonic (Weyers, Paterson, 2001; Calabrese, Blaine, 2005).
It is known that any stressful environmental factors cause an increase in the production of active oxygen forms, which leads to an increase in the process of peroxidation in cell membranes (Kamyshnikov, 2002). Based on our data, we can conclude that the used concentrations of A. chroococcum did not cause stress in wheat seedlings.
Based on the above, we can conclude that the studied strain of A. chroococcum can directly affect the germination of seeds and the state of seedlings of T. aestivum. However, its ability to change the studied parameters of T. aestivum depends on the number of nitrogen fixer cells introduced into the nutrient solution, as well as on the type of indicator. Thus, A. chroococcum in all studied concentrations did not affect the biochemical parameters (the intensity of lipid peroxidation and the content of chlorophylls and carotenoids in the leaf), it reduced seed germination and led to an increase in the biomass of seedlings. At the same time, changes in the concentration of A. chroococcum cells introduced into the nutrient solution caused a non-monotonic response of the raw biomass of the shoot and the root system of T. aestivum. Apparently, the effects on germination and biomass are mainly related to the effects of auxins synthesized by the nitrogen fixer, since these phytohormones can stimulate growth and inhibit the germination process. In addition, it is known that the "dose – effect" relationship for auxins can be non-monotonic. The results of the study can serve as a basis for developing ideas about the specifics of biotic relationships between the studied species, as well as improving methods of pre-sowing treatment of T. aestivum seeds.
Conclusions
Based on the above, we can conclude that the studied strain of A. chroococcum can directly affect the germination of seeds and the state of seedlings of T. aestivum. However, its ability to change the studied parameters of T. aestivum depends on the number of nitrogen fixer cells introduced into the nutrient solution, as well as on the type of indicator. Thus, A. chroococcum in all studied concentrations did not affect the biochemical parameters (the intensity of lipid peroxidation and the content of chlorophylls and carotenoids in the leaf), it reduced seed germination and led to an increase in the biomass of seedlings. At the same time, changes in the concentration of A. chroococcum cells introduced into the nutrient solution caused a non-monotonic response of the raw biomass of the shoot and the root system of T. aestivum. Apparently, the effects on germination and biomass are mainly related to the effects of auxins synthesized by the nitrogen fixer, since these phytohormones can stimulate growth and inhibit the germination process. In addition, it is known that the "dose – effect" relationship for auxins can be non-monotonic. The results of the study can serve as a basis for developing ideas about the specifics of biotic relationships between the studied species, as well as improving methods of pre-sowing treatment of T. aestivum seeds.
References
Erofeeva E. A. Hormesis and paradoxical effects of wheat seedling (Triticum aestivum L.) parameters upon exposure to different pollutants in a wide range of doses, Dose Response. 2014. Vol. 12. No 1. P. 121–135.
Feoktistova N. V. Mardanova A. M. Hadieva G. F. Sharipova M. R. Rhizosphere bacteria, Uchenye zapiski Kazanskogo universiteta. Ser. Estestvennye nauki. 2016. T. 158. Kn. 2. P. 207–224.
Howard J. B., Rees D. C. How many metals does it take to fix N2? A mechanistic overview of biological nitrogen fixation, Proceedings of the National Academy of Sciences of the United States of America. 2006. Vol. 103. No 46. P. 17088–17093.
Kamyshnikov V. S. Reference book on clinical and biochemical laboratory diagnostics. Minsk: Belarus', 2002. T. 2. 495 p.
Kirichenko E. Titova L. Koc' S. The bacterization effectiveness of spring wheat seeds with a new strain of Аzotobacter chroococcum T76, Stiinta Agricola. 2010. No. 1. P. 21–24.
Kirichenko E. V. Koc' S. Ya. The use of Azotobacter chroococcum to create complex biological preparations, Biotehnologiya. 2011. T. 4. No. 3. P. 74–81.
Kirichenko E. V. Biological activity of spring wheat rhizosphere soil in association with Azotobacter chroococcum T79 bacteria modified with N-acetyl-D-glucosamine, Mikrobiologiya i biotehnologiya. 2016. No. 3. P. 30–42.
Nefed'eva E. E. Belopuhov S. L. Verhoturov V. V. Lysak V. I. The role of phytohormones in the regulation of seed germination, Izvestiya vuzov. Prikladnaya biohimiya i biotehnologiya. 2013. No. 1. P. 61–65.
Salabrese E. J., Blain R. B. The occurrence of hormetic dose responses in the toxicological literature, the hormesis database: an overview, Toxicology and Applied Pharmacology. 2005. Vol. 202. P. 1451–1474.
Shlyk A. A. Determination of chlorophylls and carotenoids in green leaf extracts, Biohimicheskie metody v fiziologii rasteniy. M.: Nauka, 1971. P. 154–170.
Wani S. A., Chand S., Ali T. Potential use of Azotobacter chroococcum in crop production, Current Agriculture Research Journal. 2013. Vol. 1. No 1. P. 35–38.
Weyers J. D., Paterson N. W. Plant hormones and the control of physiological processes, New Phytol. 2001. Vol. 152. P. 375–407.





 © 2011 - 2026
© 2011 - 2026