Introduction
The study of the species composition and biotopic distribution of malaria mosquitoes in the Upper Volga Lowlands is of great theoretical and practical interest, given the epidemiological importance of Anopheles mosquitoes as vectors of vector-borne diseases of humans and animals. Past studies in the vast Upper Volga region were conducted during the period of malaria control in the 30-50s of the XX century (Egorov, 2011). The data on the fauna and ecology of malaria mosquitoes obtained in these years need to be revised due to the description of a number of new sibling species of the Maculipennis complex. The current species list of the mosquito fauna from the Upper Volga region includes only two species of Anopheles: An. messeae Fal, 1926; An. claviger Meig., 1804 (Smirnov et al., 2006; Egorov, 2011). There are no data on other sibling species of the Maculipennis complex besides An. messeae. This is due to the need for modern cytogenetic or molecular genetic methods of species diagnosis. Our study of malarial mosquitoes of the Upper Volga lowlands is intended to fill this gap.
The Upper Volga sandr-alluvial lowland is located in the sub-taiga natural zone. The habitats of a number of interzonal species of malaria mosquitoes overlap in this zone. The Upper Volga lowland extends in the central part of the East European Plain and is located in the territory of the Moscow, Tver and Yaroslavl Regions. The relief of the lowland was formed by the Moscow glaciation; it is flat, with separate hills and waterlogged depressions. The territory of the Upper Volga lowland is bordered by a group of hills and ridges over 200 m high: Klinsko-Dmitrovskaya ridge, Likhoslavl ridge, Bezhetsky top, Tverskaya ridge (Wagner, Manucharyants, 2003; National Atlas..., 2004). The lowland-marsh hydrolandscape is responsible for the great diversity of mosquito breeding sites. The biotopic distribution of closely related Anopheles species living in sympatric conditions and having overlapping ecological niches is of particular interest. First of all, this refers to the sibling species of malaria mosquitoes of the Maculipennis complex. Species of this group are able to develop in the same larval habitats, and changes in the relative abundance of mosquitoes in space and time may serve as an indicator of ecological specialisation of these closely related species. The aim of this work was to determine the species composition, relative abundance and biotopic distribution of malaria mosquito larvae in the habitats of the Upper Volga lowland and adjacent uplands.
Materials
4th instar Anopheles larvae were collected in 2013-2021 from 13 habitats in the Upper Volga lowland and 6 habitats on adjacent uplands and ridges. Material was collected in the following habitats in the Moscow Region: Sergiev Posad urban district - in Skoropuskovsky settlement (56.371336, 38.142528); Taldom urban district - in biotopes of the reserve "Crane Homeland" in v. Kostenevo (56.725472, 37.770389, v. Kunilovo, (56.730528, 37.757917), v. Aibutovo (56.730528, 37.757917), v. Dmitrovka (56.750167, 37. 753944), v. Kostolygino (56.722417, 37.866972), and in two biotopes of Verbilki settlement (56.540160, 37.585839; 56.540042, 37.588119). Malaria mosquitoes were sampled in the following habitats in Tver Region: Rzhev Municipal District - in v. Gorki of Itomlya Rural Settlement (56. 455049, 33.891225); in the city of Tver (56.797623, 36.043067); in the Kalinin municipal district – in Chupriyanovka settlement (56.751500, 36.041028), in the village of Staroye Bryantsevo (56.898383, 35.796668); Konakovo municipal district - in Redkino settlement (56.638194, 36.294667); Likhoslavl municipal district - in Baranovka village (57.202067, 35.340563), in Priozerny settlement (57.130333, 35. 495694); Spirov Municipal District - in the v. Spirovo (57.433425, 34.983388); in the town of Kashin (57.358139, 37.595000); Kalyazin Municipal District - in the v. Chigiryovo (57.262528, 37.911056); in the town of Bezhetsk (57.753194, 36.697861); Sonkovo municipal district - in the village of Novye Goritsy (57.770806, 37.200167). Larvae were collected with a medical cuvette from the water surface in permanent water ponds with abundant coastal aquatic vegetation.
Methods
Larvae captured in water bodies were fixed in an alcohol-acetic mixture prepared at a ratio of 3:1. Paired salivary glands were extracted under a MBS-10 stereoscopic microscope. The species affiliation of malaria mosquitoes was determined by morphological characters (Gutsevich et al., 1970; Zvantsov et al., 2003) and the pattern of polytene chromosome bands. Temporary preparations of polytene chromosomes were obtained from isolated salivary glands of mosquitoes. The chromosomes were stained with 2% lactoacetorrsein according to standard methods (Perevozkin, 2007). The species composition of malaria mosquitoes was determined by comparing polytene chromosomes of larvae with photomaps of karyotypes of Palaearctic Anopheles species (Stegnii & Kabanova, 1978; Artemov et al., 2018, 2021). Malaria mosquitoes Anopheles messeae s. l. include two cryptic species: An. daciae Linton, Nicolescu & Harbach, 2004 and An. messeae Fall., 1926. Homo- and heterozygous chromosomal rearrangements diagnostic for these species were recorded in the karyotypes of An. messeae s. l. larvae (Naumenko et al., 2020; Brusentsov et al., 2023). Homo- heterozygotes with inversion of sex chromosome XL0 is found exclusively in An. daciae, alternative inversion XL1 is present in both species. Homo- heterozygotes with autosomal inversion 2R1 are present in populations of An. messeae s. s. Homozygotes 2R00 are present in both species. Interspecific hybrids An. daciae × An. messeaeae were identified by the simultaneous presence of heterozygotes for both inversions XL0 and 2R1 in the karyotypes. It was previously shown that the frequencies of homo- and heterozygotes for inversions in An. daciae and An. messeae populations, with some exceptions, do not deviate significantly from those expected according to the Hardy-Weinberg equation (Brusentsov et al., 2023). For species separation, the number of An. messeae individuals with 2R00 homozygotes was estimated based on the Hardy-Weinberg equation using the formula:
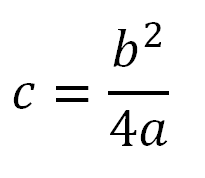
,
where c is the number of homozygotes 2R00 (corresponds to the frequency p2 × N); b is the number of heterozygotes 2R01 (corresponds to the frequency 2pq × N); a is the number of homozygotes 2R11 (corresponds to the frequency q2 × N); p and q are the frequencies of inversions 2R0 and 2R1; N is the total number of individuals in the sample.
The estimated frequency of An. messeae s. s. mosquitoes was determined as the ratio of the sum of individuals with these genotypes (a+b+c) to the total number of all An. messeae s. l. individuals in the sample. The estimated value (c) may be overestimated in individual samples in case of excess heterozygotes in local populations. It was taken into account that the estimated number of all individuals of An. messeae s. s. together with An. daciae and interspecific hybrids in such populations cannot exceed the number of all individuals in the sample.
Karyotypes were determined in 3211 larvae.
Results
The results of cytogenetic analysis of malaria mosquito samples showed that all known species of the Anopheles fauna of the northern part of the Russian Plain inhabit the territory of the Upper Volga lowland: An. messeae Fall., 1926; An. maculipennis Meig., 1818; An. beklemishevi Stegn. et Kabanova, 1976; An. claviger Meig., 1804 (Table 1). An. messeae s. l. larvae were dominant in all studied brood sites, except for one biotope where only An. claviger was detected (sample No. 22). This biotope differs from typical breeding sites of Maculipennis mosquitoes. Larvae were captured in two ditches (20-30 cm wide, 5-12 cm deep, up to 20 meters long) with running spring water.
An. beklemishevi was found in the southern part of the Upper Volga Lowland in two biotopes of the "Crane Homeland" reserve; in a biotope located in Verbilki settlement, as well as in two habitats in the adjacent areas of the Valdai Upland and Likhoslavl Ridge (Fig. 1). The proportion of An. beklemishevi among other larvae in these habitats was 0.7-2.6%.
An. maculipennis mosquitoes were found in 12 habitats both in the Upper Volga Lowland and, especially, on adjacent uplands and ridges (Fig. 1). Larvae of this species developed together with An. messeae s. l. The proportion of An. maculipennis individuals in permanent larval habitats varied from 1 to 37%. The maximum dominance indices of this species were observed in biotopes of the Klinsko-Dmitrovskaya Ridge (37.0±5.4%; sample No. 1), and also in the area of the Likhoslavl Ridge (28.7±.3% and 24.2±5.4%; samples No. 27 and 29, respectively).
The larvae of An. messeae s. l. dominated in the malaria mosquito brood sites we studied in the Upper Volga lowland and surrounding uplands. The proportion of An. messeae s. l. ranged from 63 to 100%.

Fig. 1. Species composition of malaria mosquitoes in larval collection sites in the Upper Volga Lowland and adjacent uplands.
Table 1. Species composition of malaria mosquitoes in the studied biotopes of the Upper Volga lowland and adjacent territories
| Biotopes | Collection Date | Species composition (number of individuals) | |||
| An. messeae s. l. | An. maculipennis s. s. | An. beklemishevi | An. claviger | ||
| Moscow Region | |||||
| Skoropuskovsky settlement, pond | 21.07.2016 | 51 | 30 | 0 | 0 |
| Kostenevo village, pond | 11.06.2019 - 04.07.2020 | 275 | 0 | 0 | 0 |
| Kunilovo village, pond
|
12.06.2019 - 30.07.2019 | 265 | 0 | 0 | 0 |
| Aibutovo village, pond | 13.06.2019 - 31.07.2020 | 452 | 0 | 0 | 0 |
| Dmitrovka village, pond | 26.06.2019 | 128 | 0 | 1 | 0 |
| Kostolygino village, pond | 31.07.2019 - 23.05.2021 | 487 | 2 | 5 | 0 |
| Verbilki settlement, quarry | 05.08.2020 - 03.08.2021 | 251 | 1 | 1 | 0 |
| Verbilki settlement, spring | 03.08.2021 | 0 | 0 | 0 | 130 |
| Tver Region | |||||
| Gorki village, Volga River backwaters | 05.08.2013 | 119 | 2 | 1 | 0 |
| Tver city, pond | 21.08.2016 | 120 | 1 | 0 | 0 |
| Chupriyanovka settlement, pond | 21.06.2020 | 98 | 2 | 0 | 0 |
| Redkino settlement, pond | 22.06.2020 | 51 | 0 | 0 | 0 |
| Staroye Bryantsevo village, pond | 22.06.2020 | 77 | 31 | 0 | 0 |
| Baranovka village, pond | 02.07.2021 | 80 | 15 | 0 | 0 |
| Spirovo village, pond | 03.07.2021 | 46 | 15 | 1 | 0 |
| Kashin town, pond | 31.07.2021 | 101 | 0 | 0 | 0 |
| Chigiryovo village, pond | 30.07.2021 | 97 | 7 | 0 | 0 |
| Priozerny settlement, lake | 04.07.2021 | 101 | 4 | 0 | 0 |
| Bezhetsk town, pond | 13.08.2021 | 65 | 0 | 0 | 0 |
| Novye Goritsy village, pond | 14.08.2021 | 94 | 4 | 0 | 0 |
The presence of preimaginal stages of An. messeae s. l. mosquitoes is conditioned by certain ecological parameters of breeding sites. The following range of ecological parameters is characteristic of permanent water bodies in which An. messeae s. l. mosquitoes were found. The hydrogen index ranges from neutral to slightly alkaline - 7.22-8.85. The electrical conductivity values of microsiemens per centimeter (μs) and total minerals and salts in water (ppm) ranged from 156-550 and 78-275, respectively. All water bodies in which An. messeae s. l. larvae were found were characterized by similar composition of aquatic vegetation. The following plants grew in these water bodies: Ceratophyllum demersum L., Myriophyllum spicatum L., Typha latifolia L., Potamogeton natans L., Alisma plantago-aquatica L., Elodea canadensis Michx., Equisetum fluviatile L., Eleocharis palustris L., Hydrocharis morsus-ranae L., Lemna sp. L., Oenanthe aquatica L., Schoenoplectus lacustris L., Galium palustre L. High larval densities were found in clusters of filamentous algae of the genus Spirogyra. During the breeding season, the stability of the ecological characteristics of water bodies can be disturbed under the influence of both endogenous causes and anthropogenic factors. The most significant role in changing the suitability of water bodies for larvae is played by the transformation of aquatic and near-water vegetation. Decrease in the density of Anopheles larvae was repeatedly observed when the abundance of free-floating plants (Lemna ssp., Cladophora ssp., etc.) actively increased in water bodies and the water surface free of plants sharply decreased. In one of the control reservoirs of the Crane Homeland Reserve, we observed the disappearance of An. messeae s. l. larvae after the banks were mowed, resulting in the disappearance of almost all representatives of free-floating and rooting plants.
Based on cytogenetic analysis, we found that in the studied habitats An. messeae s. l. mosquitoes are represented by two sibling species: An. daciae and An. messeae s. s. The estimated proportion of An. messeae s. s. larvae in all biotopes varies in the range of 57.5-96.9%, indicating the dominance of mosquitoes of this species.
The study of the chromosomal composition of larvae allowed us to determine the level of interspecific hybridization between An. daciae and An. messeae s. s. Interspecific hybrids An. daciae × An. messeae were found in 16 of 19 larval biotopes studied. The proportion of interspecific hybrids in the biotopes ranged from 0.4 to 7.8% (Table 2).
Table 2. Proportion of An. daciae, An. messeae s. s. and their hybrids among An. messeae s. l. larvae in habitats of the Upper Volga Lowland and adjacent uplands
| Habitat (sample numbers) | Number of specimens | Estimated frequencies of species and their hybrids, f ± Sf (%) | ||
| An. daciae | An. messeae s. s. | Hybrids | ||
| Skoropuskovsky (No.1) | 51 | 13.7 ± 4.8 | 82.3 ± 5.3 | 4.0 ± 2.7 |
| Kostenevo (No.2-4) | 275 | 16.4 ± 2.2 | 83.6 ± 2.2 | 0 |
| Kunilovo (№5-7) | 265 | 16.6 ± 2.3 | 82.6 ± 2.3 | 0.8 ± 0.5 |
| Aibutovo (No.8-13) | 452 | 19.2 ± 1.9 | 78.8 ± 1.9 | 2.0 ± 0.7 |
| Dmitrovka (No.14) | 128 | 18.7 ± 3.5 | 80.5 ± 3.5 | 0.8 ± 0.8 |
| Kostolygino (No.15-19) | 487 | 15.4 ± 2.7 | 82.5 ± 1.7 | 2.1 ± 0.6 |
| Verbilki (No.20-21) | 251 | 31.9 ± 2.9 | 66.9 ± 3.0 | 1.2 ± 0.7 |
| Gorki (No.23) | 119 | 17.6 ± 3.5 | 79.0 ± 3.7 | 3.4 ± 1.7 |
| Tver (No.24) | 120 | 25.0 ± 4.0 | 70.8 ± 4.2 | 4.2 ± 1.8 |
| Chupriyanovka (No.25) | 98 | 3.1 ± 1.8 | 96.9 ± 1.8 | 0 |
| Redkino (No.26) | 51 | 21.5 ± 5.8 | 70.7 ± 6.4 | 7.8 ± 3.8 |
| Staroe Bryancevo (No.27) | 77 | 26.0 ± 5.0 | 72.7 ± 5.1 | 1.3 ± 1.3 |
| Baranovka (No.28) | 80 | 33.8 ± 5.3 | 59.9 ± 5.5 | 6.3 ± 2.7 |
| Spirovo (No.29) | 46 | 13.0 ± 5.0 | 87.0 ± 5.0 | 0 |
| Kashin (No.30) | 101 | 14.8 ± 3.5 | 81.2 ± 3.9 | 4.0 ± 1.9 |
| Chigirevo (No.31) | 97 | 10.3 ± 3.1 | 85.6 ± 3.6 | 4.1 ± 2.0 |
| Priozernyj (No.32) | 101 | 28.7 ± 4.5 | 68.3 ± 4.6 | 3.0 ± 1.7 |
| Bezhetsk (No.33) | 65 | 12.3 ± 4.1 | 86.2 ± 4.3 | 1.5 ± 1.5 |
| Novye Goritsy (No.34) | 94 | 18.0 ± 4.0 | 80.9 ± 4.1 | 1.1 ± 1 |
Discussion
The data obtained allow us to clarify the species composition of malaria mosquitoes inhabiting the Upper Volga Lowlands. Species An. beklemishevi, An. daciae and An. maculipennis were not included in the existing species list of the Upper Volga region (Egorov, 2011). For the first time, the mosquito breeding sites of these species were established. It is shown that larval development of sibling species of Maculipennis complex in the study area occurs in the same water bodies. At the same time, the peculiarities of biotopic distribution of different species of malarial mosquitoes were revealed.
An. claviger was found in a groundwater outfall in the vicinity of Verbilki (Taldomskiy urban district, Moscow region). Since this species prefers cold freshwater bodies with running water, cohabitation of An. claviger with species of the Maculipennis complex is extremely rare.
An. beklemishevi is the northernmost species among malaria mosquitoes. This species inhabits wetlands in the zone of coniferous and mixed forests (Novikov, 2016; Soboleva et al., 2020). The southernmost habitats of this species were found in the Meshcherskaya lowland (Lopatin et al., 2020). An. beklemishevi is confined to specific habitats, its range is not continuous. Biotopes where An. beklemishevi was found are characterized by increased shading and relatively low density of malaria mosquito larvae. For example, the water body in Dmitrovka village (sample No. 14) is surrounded by a continuous ring of tree and shrub vegetation. The shore of the pond in the Kostolygino village (samples No.17-18), where larvae were collected, is also shaded by willow bushes Salix spp. and broad-leaved hornwort Typha latifolia L., 1753, growing in large numbers along the shoreline. Previously, the ecological preferences of co-occurring mosquitoes An. beklemishevi and An. messeae s. l. were investigated in different larval habitats in the floodplain of the Chulym River in the south of Western Siberia (Perevozkin et al., 2009). An. beklemishevi larvae were found with increased frequency in shallow shaded water bodies. Mosquitoes An. messeae s. l. dominated in open warmed water bodies. The available data on the geographical distribution of An. beklemishevi indicate that the southern part of the range of this species in the sub-taiga zone of the Russian Plain has a fragmentary character, the marginal populations are small and partially isolated from each other.
The malaria mosquito An. maculipennis occurs with high frequency in habitats in the center and south of the Russian Plain (Stegnii, 1991). Apparently, the spread of this species to the north of the taiga zone is hindered by low wintering temperatures combined with a short reproductive season. Currently, under the conditions of climate warming, the range of this species is expanding northward and eastward to the Middle and Southern Urals (Novikov and Vaulin, 2014). According to our data, in Karelia the species has moved up to the 64th parallel. The northernmost population of this species was found in the vicinity of Kem (Perevozkin et al., 2012). In addition to a combination of abiotic factors, the advance of An. maculipennis to the north and east of the Palaearctic may be hindered by competition with An. messeae s. l. mosquitoes. An. messeae s. l. mosquitoes belong to polyzonal species and are widely distributed in different landscape and climatic zones of Eurasia. It is no coincidence that An. messeae s. l. mosquitoes have the highest level of chromosomal polymorphism compared to other representatives of the Maculipennis group (Stegnii et al., 2016).
The results of our cytogenetic analysis showed that An. messeae s. l. mosquitoes are represented by two sibling species with incomplete reproductive isolation, An. daciae and An. messeae s. s. The habitats of these sibling species overlap in the center of the Russian Plain (Naumenko et al., 2020). We found that both species occur in all biotopes of the Upper Volga Lowland and adjacent territories, where malaria mosquitoes were mass-reared (Table 2). Only molecular genetic analysis provides precise data on the ratio of the two species in the jointly inhabited biotopes. The main taxonomic feature is the nucleotide composition of the second internal transcribed spacer ITS2 of the cluster of ribosomal DNA: 5 positions were found, which differ between the two species (Nicolescu et al., 2004; Naumenko et al., 2020). Molecular genetic diagnosis is complicated by polymorphism at 3 of these positions, which was found in An. daciae (Brusentsov et al., 2023). In our opinion, the results of chromosomal variability analysis can be used to determine the geographic distribution of An. daciae and An. messeae s. s. sibling species. Comparison of data from molecular genetic and cytogenetic analysis showed that both species are chromosomally polymorphic (Brusentsov et al., 2023). There are common and species-specific inversions in the populations of both species. The XL0 inversion occurs with high frequency in An. daciae, but is practically absent in An. messeae s. s. On the other hand, autosomal 2R1 inversion is widely represented in An. messeae s. s. populations in the north and center of the species range, but is extremely rare in An. daciae populations (apparently, only in heterozygotes in interspecific hybrids). The presence of homo- and heterozygotes for inversions XL0 and 2R1 proves that both species are present in all water bodies where An. messeae s. l. larvae are present. The high frequency of homo- and heterozygotes 2R11 and 2R01 (above 50% in all biotopes except Baranovka and separate samples in Verbilki and Kostenevo) indicates the dominance of An. messeae s. s. over An. daciae. Among homozygotes with XL1 and 2R0 inversions, some mosquitoes belong to An. messeae s. s. and some to An. daciae. It is possible to determine the species status of these larvae only by molecular genetic methods. But it is possible to calculate the proportion of 2R00 homozygotes of An. messeae s. s. using the Hardy-Weinberg equation, which is the starting point for analyzing the genetic structure of populations (Zhivotovsky, 2021). It was previously shown that in most populations of An. messeae s. s. living in sympatry with An. daciae, there are no significant deviations of inversion genotype frequencies from the Hardy-Weinberg ratio (Brusentsov et al., 2023). Knowing the number of heterozygotes 2R01 and homozygotes 2R11, we can give a probabilistic estimate of the number of homozygotes 2R00 in An. messeae s. s. using the above formula. The 2R00 homozygote frequencies we calculated allowed us to determine the ratio of An. messeae s. s. and An. daciae in larval habitats (Table 2). It should be noted that in some samples the estimated number of 2R00 homozygotes was overestimated because the sum of individuals of both species exceeds the sample size. In these cases, the number of 2R00 homozygotes was limited so that the total frequency of An. messeae s. s., An. daciae and their hybrids did not exceed 100%. Some exceeding of the estimated number of 2R00 homozygotes in An. messeae s. s. may be due to sampling error or heterozygote excess due to population dynamics factors (e.g., the effect of overdominance in local populations).
The data obtained indicate that interspecific hybridization occurs in most habitats of the Upper Volga Lowland, which supports the conclusion that An. daciae and An. messeae are incompletely reproductively isolated and that there is genetic introgression in these sibling species (Brusentsov et al., 2023). The results of full genome sequencing of An. daciae and An. messeae s. s. showed that up to 20% of individuals of both species in sympatry zones are of hybrid origin. At the same time, comparison of genomes indicates profound differences in the nucleotide composition of sex chromosomes of An. daciae and An. messeae s. s., which ensures the isolation of their gene pools (Naumenko et al., 2020).
Conclusions
As a result of our studies, we have determined the species composition of malaria mosquitoes in the biotopes of the Upper Volga lowland and surrounding ridges and hills. The malaria mosquito fauna includes five species: An. beklemishevi, An. claviger, An. daciae, An. maculipennis s. s., An. messeae s. s. The mosquito An. claviger is a highly specialized species, inhabits spring-type water bodies and is ecologically isolated from other malaria mosquitoes. Four cryptic species of the Maculipennis complex live together in larval habitats. An. beklemishevi populations have a focal spatial distribution. The mosquitoes of this species are characterized by low relative abundance in larval habitats and prefer shaded breeding sites. An. maculipennis s. s. is a subdominant species and occurs most frequently at higher elevations. The sibling species An. messeae s. s. and An. daciae are widespread. An. messeae s. s. dominates in all jointly exploited biotopes. The absence of biotopic subdivision indicates a significant overlap between the ecological niches of An. daciae and An. messeae s. s. It is obvious that these recently separated species are at an early stage of ecological diversification. The incomplete reproductive isolation of these species testifies to the same. In fact, the entire territory of the Upper Volga Lowland is a zone of interspecific hybridization between An. daciae and An. messeae s. s.
References
Abarykova O.L. Petrov Yu.F. Mosquito fauna (Diptera, Culiddae) Eastern Upper Volga Region of the Russian Federation, Agrarnyy vestnik Urala. Seriya «Biologiya», 2006. No.2 (32). C.54-56.
Artem'ev M. M. Baranova A. M. Ganushkina L. A. Gornostaeva R. M. Darchenkova N. N. Dremova V. G. Ermishev Yu. V. Markovich N. Ya. Sergiev V. P. Malaria mosquitoes and their control on the territory of the Russian Federation: Methodological guidelines. M.: Federal'nyy centr gossanepidnadzora Minzdrava Rossii, 2000. 56 p.
Artemov G. N., Fedorova V. S., Karagodin D. A., Brusentsov I. I., Baricheva E. M., Sharakhov I. V., Gordeev M. I.,Sharakhova M. V. New Cytogenetic Photomap and Molecular Diagnostics for the Cryptic Species of the Malaria Mosquitoes Anopheles messeae and Anopheles daciae from Eurasia, Insects, 2021. 12(9):835. P. 1–16. DOI: 10.3390/insects12090835
Artemov G. N., Gordeev M. I., Kokhanenko A. A., Moskaev A. V., Velichevskaya A. I., Stegniy V. N., Sharakhov I. V., Sharakhova M.V. A standard photomap of ovarian nurse cell chromosomes and inversion polymorphism in Anopheles beklemishevi, Parasites and Vectors, 2018 V. 11(1):211. P. 1–9. DOI: 10.1186/s13071-018-2657-3
Beklemishev V. N. Ecology of the malaria mosquito. M.: Medgiz, 1944. 299 p.
Brusentsov I. I., Gordeev M. I., Yurchenko A. A., Karagodin D. A., Moskaev A. V., Hodge J. M., Burlak V. A., Artemov G. N., Sibataev A. K., Becker N., Sharakhov I. V., Baricheva E. M., Sharakhova M. V. Patterns of genetic differentiation imply distinct phylogeographic history of the mosquito species Anopheles messeae and Anopheles daciae in Eurasia, Mol Ecology, 2023. Sep 13. DOI: 10.1111/mec.17127
Egorov S. V. Species composition and structure of the fauna of blood-sucking mosquitoes (Diptera, Culicidae) in the Upper Volga region and factors determining its dynamics, Rossiyskiy parazitologicheskiy zhurnal. 2011 No. 1 P. 15-17.
Gucevich A. M. Monchadskiy A. S. Shtakel'berg A. A. SR. Mosquitoes. The family Culicidae. Fauna of the USSR. Diptera insects.. L.: Nauka, 1970. T. 3. Vyp. 4. 384 p.
Lopatin A. A. Panov V. I. Moskaev A. V. Gordeev M. I. Study of the species and chromosomal composition of populations of malaria mosquitoes of the Ryazan Meschera, Materialy Mezhdunarodnoy nauchnoy onlayn-konferencii molodyh uchenyh «Nauka na blago chelovechestva – 2020» (Moskva, 20–24 aprelya 2020 g.). M.: MGOU, 2020. P. 251–256.
Naumenko A. N., Karagodin D. A., Yurchenko A. A., Moskaev A. V., Martin O. I., Baricheva E. M., Sharakhov I. V., Gordeev M. I., Sharakhova M. V. Chromosome and Genome Divergence between the Cryptic Eurasian Malaria Vector-Species Anopheles messeae and Anopheles daciae, Genes, 2020. 11(2): 165. P. 1–22. DOI: 10.3390/genes11020165
Nicolescu G., Linton Y. M., Vladimirescu A., Howard T. M., Harbach R. E. Mosquitoes of the Anopheles maculipennis group (Diptera: Culicidae) in Romania, with the discovery and formal recognition of a new species based on molecular and morphological evidence. Bull. Entomol. Res., 2004. 94: P. 525–535. DOI: 10.1079/ber2004330
Novikov Y. M. On the ecology and range of Anopheles beklemishevi (Diptera: Culicidae) with reference to the taxonomy of An. lewisi, Journal of Vector Ecology, 2016. T. 41. No.. 2 P. 204–214. DOI: 10.1111/jvec.12215
Novikov Y., Vaulin O. Expansion of Anopheles maculipennis s. s. (Diptera: Culicidae) to northeastern Europe and northwestern Asia: Causes and Consequences. Parasites & vectors, 2014. V.7 (389). P. 1–10. DOI: 10.1186/1756-3305-7-389.
Overview of weather conditions in the European region for May 8-11, 2021, Rosgidromet. URL: https://www.meteorf.gov.ru/press/news/24534/?sphrase_id=791819 (data obrascheniya: 14.11.2023).
Perevozkin V. P. Gordeev M. I. Bondarchuk S. S. Chromosomal polymorphism and patterns of formation of the subpopulation organization of malaria mosquitoes Anopheles (Diptera, Culicidae) in the habitats of the Tomsk region, Genetika, 2009. T. 45. No.. 4. P. 478–487. DOI: 10.1134/S102279540904005X
Perevozkin V. P. Gordeev M. I. Moskaev A. V. Ahmetova N. M. Bondarchuk S. S. Distribution and inversion polymorphism of malaria mosquitoes of Karelia// Genetika, 2012. T. 48. No..7.S 806-806.
Perevozkin V. P. Adaptive polymorphism of malaria mosquitoes of the Anopheles maculipennis complex, Nauchno-prakticheskoe rukovodstvo po malyarii (epidemiologiya, sistematika, genetika). Tomsk: Izd-vo Tom. un-ta. 2007. P. 105–145.
Soboleva E. S. Burlak V. A. Sharahova M. V. Artemov G. N. Inversion polymorphism of natural populations of Anopheles beklemishevi Stegnii et Kabanova in Western Siberia, Konceptual'nye i prikladnye aspekty nauchnyh issledovaniy i obrazovaniya v oblasti zoologii bespozvonochnyh: sbornik statey V Mezhdunarodnoy konferencii, 26-28 oktyabrya 2020 g., g. Tomsk, Rossiya. Tomsk, 2020. P. 140–144.
Stegniy V. N. Kabanova V. M. Chromosomal analysis of malaria mosquitoes Anopheles atroparvus and A. maculipennis (Diptera, Culicidae), Zoologicheskiy zhurnal, 1978. T. 57. No.. 4. P. 613–619.
Stegniy V. N. Pischelko A. O. Sibataev A. K. Abylkasymova G. M. Spatiotemporal changes in the frequency of chromosomal inversions over the range of the malaria mosquito Anopheles messeae Fall. (Culicidae) over a 40-year monitoring period, Genetika, 2016. T. 52. No.. 6. P. 664–664. DOI: 10.7868/S0016675816060138
Stegniy V. N. Population genetics and evolution of malaria mosquitoes. Tomsk: Izd-vo Tomskogo un-ta, 1991. 136 p.
The National Atlas of Russia: in 4 volumes. Vol. 1. General characteristics of the territory, Otv. red.: G.V. Pozdnyak, N.N. Polunkina, N.V. Smurova; redkol. V.F. Habarov i dr. M.: Roskartografiya, 2004. 496 p.
Vagner B. B. Manucharyanc B. O. Geology, relief and minerals of the Moscow region. M.: MGPU, 2003. 81 p.
Zhivotovskiy L. A. Genetics of natural populations. Yoshkar-Ola: «Vertikal'», 2021. 600 p.
Zvancov A. B. Ezhov M. N. Artem'ev M. M. Malaria vectors (Diptera, Culicidae, Anopheles) The Commonwealth of Independent States (CIS). Kopengagen: VOZ, 2003. 312 p.
Acknowledgements
The study was funded by RNF grant No. 22-24-00183 "Chromosomal polymorphism in populations of sibling species of malaria mosquitoes of the taiga zone of Eurasia", https://rscf.ru/project/22-24-00183/.





 © 2011 - 2026
© 2011 - 2026