Introduction
The scallop Mizuhopecten yessoensis is a bivalve mollusk, the representative of the family Pectinidae, it lives in the waters of the Sea of Japan. This species is a unique object due to its ability to accumulate cadmium in soft tissues even in habitats where cadmium does not exceed background values (Chelomin, Belcheva, 1991; Chelomin et al., 1995).
The phenomenon of resistance to cadmium is well known in the world literature and is explained by the functioning of special cadmium-binding proteins metallothioneins (MT) in tissues. Metallothioneins are the class of low-molecular proteins (6-7 kDa) found in mammals. These proteins, in the amino acid composition of which there is up to 30% cysteine, belong to the classic MT. Synthesis of these proteins is induced when vital Cu and Zn enter the body, and in response to the arrival of Cd, Hg and a number of other toxic metals. However, wide distribution of metallothioneins does not mean that it is the only protein group involved in the binding and detoxification of metals, and above all cadmium. In some organisms different Cd-binding proteins with variations in molecular weight, cysteine amount and amino acid composition were found. Similar characteristics do not allow attributing these proteins to MT classical type, and therefore they are called M-like proteins (Ponzano, 2001; Stone, 1986; Uthe, Chou, 1987).
We have previously established (Zhukovskaya et al., 2012) that in the digestive gland of adult individuals (5-7 years) of the scallop Mizuhopecten yessoensis two MT-like proteins with molecular weights of 72 and 43 kDa are responsible for cadmium binding. The purpose of the research is to reveal the features of adaptation of 1-, 2- and 3-year old individuals of the scallop Mizuhopecten yessoensis to cadmium.
Materials
Individuals of the scallop Mizuhopecten yessoensis were selected from the Severnaya Bay (42 93' N, 131 40' E) of the Peter the Great Gulf of the Japanese Sea from the territory of mariculture farm (Fig. 1) in June. Three groups of scallops: 1-, 2- and 3-year-old individuals were taken. Each age sample consisted of 40 individuals. After catching mollusks were transported to aquariums to carry out experiments on the accumulation of cadmium.
Before the experiment the individuals of M. yessoensis were subjected to the adaptation process in aquariums with aerated running water and maintaining a constant temperature of 17ºC within 7 days. After the adaptation control scallops of each age (20) were selected, and the rest were added 300 mcg/l of CdCl2 into the media. Incubation was carried out for 10 days with a daily change of water.
Fig. 1. The map of sampling stations located in the Japanese Sea (Severnaya Bay of the Peter the Great Gulf 42 93' N, 131 40' E)
Methods
After the experiment on incubation of M. yessoensis with cadmium, further biochemical studies were performed. Isolation of MT-like cadmium-binding proteins was performed according to the MT isolation technique developed for marine invertebrates (Roesijadi et al., 1989; Thompson, Sutherland, 1992).
The digestive gland was extracted from the individuals of the seaside scallop M. yessoensis on the ice. Then it was homogenized in 0.02 M Tris-HCl buffer pH 8.5 with the presence of 1 mΜ PMSF (protease inhibition) and 10 mm DTT (to prevent oxidation of sulfhydryl groups of cysteine residues of protein amino acids). The resulting homogenate was centrifuged for 50 minutes at 10,000 rpm. The resulting supernatant (cytosol) was then processed (10 min, 75°C) to obtain a fraction of thermally stable proteins. The fraction containing thermally stable proteins was also centrifuged for 50 min at 10,000 rpm, the resulting supernatant was precipitated with 50% acetone at 4°С for 30 min at constant stirring, then the precipitate was re-dissolved and the acetone concentration was brought to 80 %. Precipitation by 80% acetone was performed at -20°C for 12 hours. The fraction containing 80 % acetone was then centrifuged (10 min at 10,000 rpm), the resulting precipitate was resuspended in 0.02 M Tris-HCl buffer pH 8.5 (fraction D).
The aliquot of resuspended precipitate (fraction D) containing thermally stable, acetone-resistant proteins was applied to a Superosa 12 column in the FPLC system at constant pressure and with a constant current of 0.02 M Tris-HCl buffer pH 8.5. Registration of the yield of fractions was performed at λ 280 nm (Shimadzu UV-1800).
Eluted fractions after FPLC containing MT-like proteins for molecular weight identification were separated by SDS-electrophoresis method in 9% polyacrylamide gel using the Lamli method (Laemli, 1970). The samples were stained by Coomassie blue. A mixture of proteins (PageRuler Prestained Protein Ladder), Fermentas (#SM0671 Lot: 00019482) were used as the standard molecular weight markers.
The content of metals (Cd, Zn, Cu) in the tissue of the digestive gland and the content of cadmium at each stage of fractionation and in chromatographic fractions was measured by an atomic-absorption spectrophotometer with flame atomization and correction of the deuterium background (Shimazu AA-6800).
The protein content at each stage of subcellular fractionation and in chromatographic fractions was determined by the Lowry method (Lowry et al., 1951).
The integral anti-radical activity of protein fractions was evaluated on the base of ability of the fractions to suppress the ABTS oxidation reaction by peroxyl and alkoxyl radicals formed during the thermal decomposition of ABAP (2, 2'-azobis (2-aminopropane) hydrochloride) (Bartosz et al., 1998). Composition of the reaction mixture: 0.1 M phosphate buffer pH 7.0, 50 mM ABTS, 200 mM ABAP. Measurements were made at a wavelength of 414 nm. The activity value was calculated using a calibration graph based on "Trolox" (a water-soluble analogue of vitamin E).
Statistically significant differences between the control and the experiment were determined using a single-component ANOVA analysis. All conclusions are based on at least 5 % of the significance level.
Results
The content of metals such as Cd, Zn, and Cu in the digestive gland tissue was studied in individuals of the seaside scallop M. yessoensis of different ages (one -, two -, and three-year-olds) (table. 1).
Table 1. Concentrations of Cd, Zn and Cu in the digestive gland of 1-, 2- and 3-year-old scallops M. yessoensis
| Age | 1 | 2 | 3 | ||||||
| Cd | Zn | Cu | Cd | Zn | Cu | Cd | Zn | Cu | |
| Control | 65±2.1 | 155±5.7 | 13±5.2 | 56±2.8 | 132±6.5 | 11±0.5 | 107±5.35 | 118±4.7 | 12±0.5 |
| Experiment | 733±36 | 165±6.4 | 17±8.5 | 283±13.5 | 95±4.75 | 13±0.45 | 321±15.5 | 113±5 | 17±0.8 |
The digestive gland of the studied age groups of M. yessoensis contains all three of the studied metals: cadmium, zinc, and copper. The content of zinc in the digestive gland of the three groups of control animals studied is higher than that of cadmium. Copper – the smallest amount. The ratio of Cd:Zn:Cu in the digestive gland of control 1+ individuals of M. yessoensis was 5:12:1, in 2+ individuals - 5:12:1, in 3+ individuals-9:10:1. Thus, in the control group of one – and two-year-old scallops, the same ability to accumulate Cd, Zn, and Cu was observed.
However, the concentrations of accumulated cadmium, zinc, and copper in control scallops are significantly higher in one-year-olds compared to two-year-olds. In three-year-old control individuals, the concentration of accumulated cadmium is twice as high, but zinc accumulates to a lesser extent compared to one - and two-year-olds. The amount of copper remains at the same level.
After incubation of seashore scallop individuals with cadmium (300 mcg/l CdCl2), the ratio of Cd:Zn:Cu in the digestive gland of the three studied age groups changed. In one-year-old individuals the ratio of Cd:Zn:Cu was 43:10:1, in two-year-old – 22:7:1, in three-year-old – 19:6,5:1. Thus, the greatest affinity to cadmium is observed in one-year-old individuals of the seaside scallop in conditions of high content of cadmium in the environment, since just in this group the highest degree of accumulation of toxic metal is observed. With increasing of age, the degree of accumulation of this metal in the digestive gland is reduced by half, from 733 to 321 mcg/g of dry weight.
1 год
Subcellular division of cadmium in the digestive gland of yearlings of the seaside scallop M. yessoensis showed that most of it is contained in the cytoplasmic cell fraction, both in control and experimental animals. The fraction containing MT-like proteins accounts for 25 % of cytoplasmic cadmium in the control and more than 33 % in the experiment.
Table 2. Cd content in subcellular fractions of the digestive gland of 1-year-old scallops M. yessoensis in control and experimental conditions (300 μg Cd/l)
| The sample's name | Control | Experiment | ||
| Homogenate | 2.8±0.1 | 100 % | 70±3.5 | 100 % |
| Cytosol | 1.9±0.09 | 68 % | 43.5±2 | 62 % |
| 80% sediment | 0.48±0.02 | 25 % | 14.5±0.07 | 33 % |
As a result of separation of the D fraction by gel-chromatography, two cadmium-containing peaks corresponding to proteins with high molecular weight were obtained. SDS- electrophoresis revealed three spots corresponding to the 120 kDa, 72 kDa, and 43 kDa regions (Fig. 2).
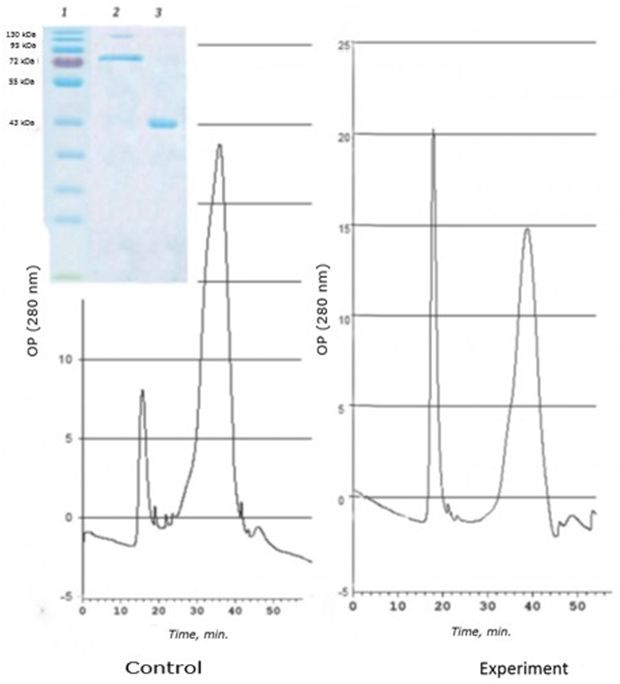
Fig. 2. Chromatographic profile (FPLC, Superosa 12, λ 280 nm) of the resuspended acetone residue (fraction D) of the digestive gland of 1-years-old scallops M. yessoensis (control and experiment – CdCl2 300 μg/l) and SDS-electrophoresis (9 %) of the eluted peaks (1 and 2). Coomassie blue stained
It was found that all three identified proteins bind toxic cadmium in both the control and experimental groups of the studied mollusks (Fig. 3).
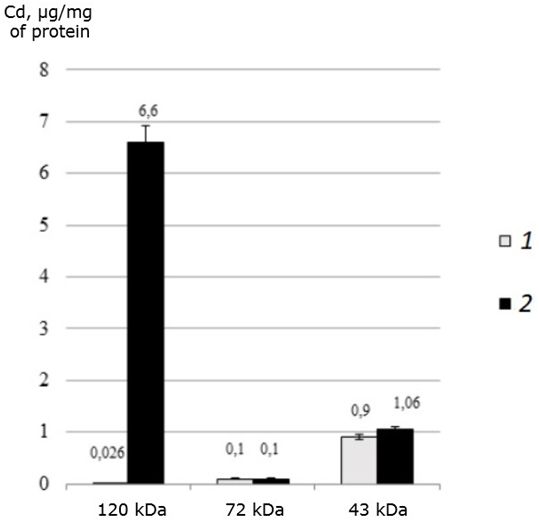
Fig. 3. The distribution of cadmium (μg/mg of protein) among the MT-like proteins of the digestive gland of one-year-old scallops M. yessoensis in control (1) and experimental (2) groups (CdCl2 300 μg/l)
It was found that in natural conditions, in one-year-old individuals of M. yessoensis cadmium is bind mainly by the 43 kDa protein. However, in individuals treated with cadmium, cadmium is redistributed from a 43 kDa protein to a 120 kDa protein. Interestingly, a protein with a molecular weight of 72 kDa did not change its affinity for cadmium, regardless of the experimental conditions.
The study of antioxidant properties showed a significant difference in values between both cadmium-binding proteins in control mollusks and in the experimental group of scallops. The results showed (Fig. 4) that in the natural habitat, cadmium-binding proteins bind accumulated cadmium and have the following AO values: for a protein of 120 kDa-86 mMol of Trolox/mg of protein, for a protein of 72 kDa – 114 mMol of Trolox/mg of protein, and for a protein of 43 kDa - 59 mMol of Trolox/mg of protein.
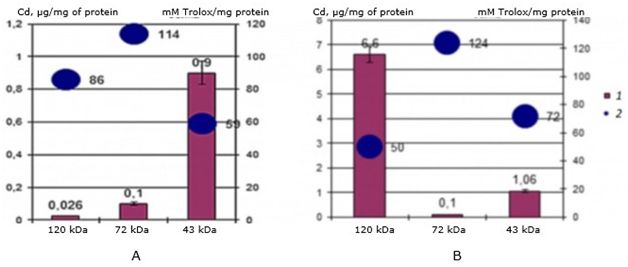
Fig. 4. AO activity (mM Trolox/mg protein) of MT-like proteins of the digestive gland of one-year-old scallops M. yessoensis in control (A) and experimental (B) groups (1 - Cd, 2 - AO activity)
Under experimental conditions, the values of antioxidant (AO) activity for the same proteins changed during the accumulation of cadmium in proteins (see Fig. 4): for a 120 kDa protein, the activity dropped to 50 mMol of Trolox/mg of protein, for a 72 kDa protein it increased to 124 mMol of Trolox/mg of protein, for a 43 kDa protein it increased to 72 mMol of Trolox/mg of protein.
The results showed that with an increase in bound cadmium in the protein, the AO activity of the protein decreases, and vice versa, with a decrease in bound cadmium – it increases. Thus, in one-year-old individuals of the seaside scallop, the protein 120 kDa performs a cadmium-binding function, and the protein 72 kDa – antioxidant, while the 48 kDa protein is responsible for binding cadmium in an environment without a cadmium load.
2 years
Subcellular division of cadmium in the digestive gland of two-year-old individuals of the seaside scallop M. yessoensis showed that most of the cadmium is contained in the membrane fraction in control individuals (10 % of cytoplasmic cadmium) and in experimental animals (20%). The fraction containing MT-like proteins accounts for 20 and 25% in the control and experiment, respectively (table. 3).
Table 3. Cd content in subcellular fractions of the digestive gland of 2-year-old scallops M. yessoensis in control and experimental conditions (300 μg Cd/l)
| The sample's name | Control | Experiment | ||
| Homogenate | 10±0.3 | 100 % | 40±2 | 100 % |
| Cytosol | 1±0.03 | 10 % | 8±0.4 | 20 % |
| 80% sediment | 0.2±0.01 | 20 % | 2±0.01 | 25 % |
A distinctive feature of this age group is a sharp decrease in the amount of high-molecular protein 120 kDa, which performs the function of binding cadmium in yearlings (Fig. 5).
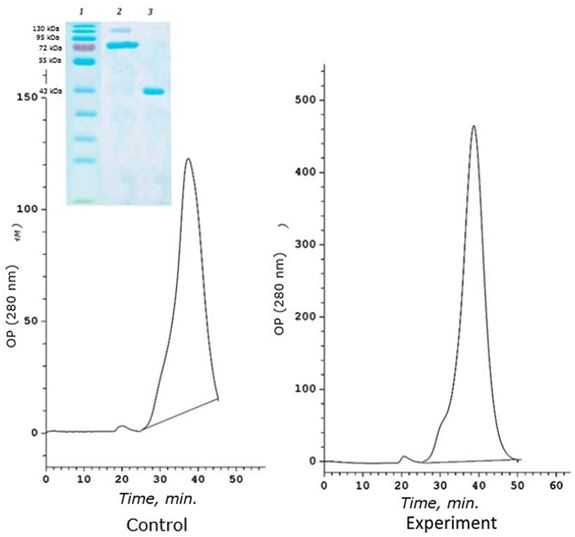
Fig. 5. Chromatographic profile (FPLC, Superosa 12, λ 280 nm) of the resuspended acetone residue (fraction D) of the digestive gland of 2-year-old scallops M. yessoensis (control and experiment – CdCl2 300 μg/l) and SDS-electrophoresis (9 %) of the eluted peaks (1 and 2). Coomassie blue
In addition, this protein does not bind cadmium at all. The main cadmium-binding protein of two-year-old individuals of the seaside scallop is 72 kDa protein (Fig. 6). This protein is responsible for binding cadmium in both control and experimental individuals of M. yessoensis. In control, the protein binds 0.18 μg Cd/mg of protein, and in experimental conditions it binds 9 times more (1.6 μg CD/mg of protein). The second identified protein with a molecular weight of 43 kDa binds a small amount of cadmium and only under experimental conditions (0.1 μg CD/mg of protein).
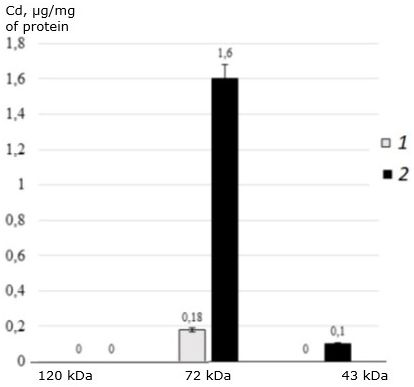
Fig. 6. The distribution of cadmium (μg/mg of protein) among the MT-like proteins of the digestive gland of two-year-old scallops M. yessoensis in control (1) and experimental (2) groups (CdCl2 300 μg/l)
Evaluation of the antioxidant activity of identified cadmium-binding proteins in two-year-old individuals of the seaside scallop M. yessoensis showed a significant difference in values between cadmium-binding proteins in the control and experimental groups of scallops. The results showed (Fig. 7) that in the natural environment, the cadmium-binding protein 120 kDa has a very low AO activity – 0.086 mMol of Trolox/mg of protein, and when incubated with cadmium, the activity increases sharply to 59.33 mMol of Trolox/mg of protein. The main cadmium-binding protein 72 kDa does not change its activity. When incubating individuals with cadmium, the activity of this digestive gland protein is only slightly reduced from 47 to 42 mMol of Trolox/mg of protein. And a more significant decrease in AO activity can be observed in the 43 kDa protein: a decrease from 114 mMol of Trolox/mg of protein in control mollusks to 53 mMol of Trolox/mg of protein in experimental scallops.
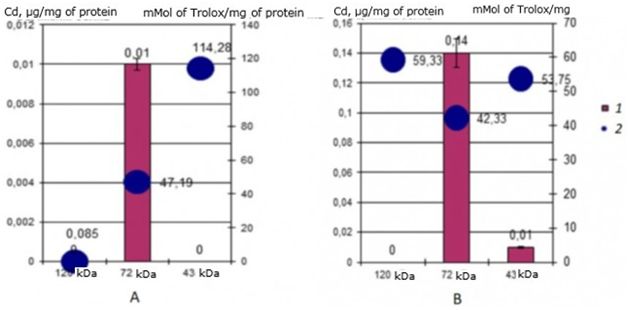
Fig. 7. AO activity (mM Trolox/mg protein) of MT-like proteins of the digestive gland of two-year-old scallops M. yessoensis in control (A) and experimental (B) groups
3 years
Subcellular division of cadmium in the digestive gland of three-year-old individuals of the seaside scallop M. yessoensis showed that half (50%) of cadmium is contained in the cytoplasmic fraction in control individuals, and 40% of the accumulated cadmium – in experimental animals. On the fraction containing MT-like proteins falls 30 and 37% of cytoplasmic cadmium in control and experiment, respectively.
Table 4. Cd content in subcellular fractions of the digestive gland 3-year-old scallops M. yessoensis in control and experimental conditions (300 μg Cd/l)
| The sample's name | Control | Experiment | ||
| Homogenate | 20±1 | 100 % | 50±2.5 | 100 % |
| Cytosol | 10±0.4 | 50 % | 20±1 | 40 % |
| 80% sediment | 3±0.12 | 30 % | 7.5±0.3 | 37 % |
A distinctive feature of this age is the complete disappearance of the 120 kDa protein from the fraction of the obtained MT-like proteins (Fig. 8). The main role in the binding of cadmium passes to the 72 kDa protein, as in the two-year-old group of seaside scallops. However, the 43 kDa protein binds cadmium (0.5 μg Cd/mg protein) in the control group of scallops in contrast to two-year-olds, in which this protein is not involved in the binding of this metal.
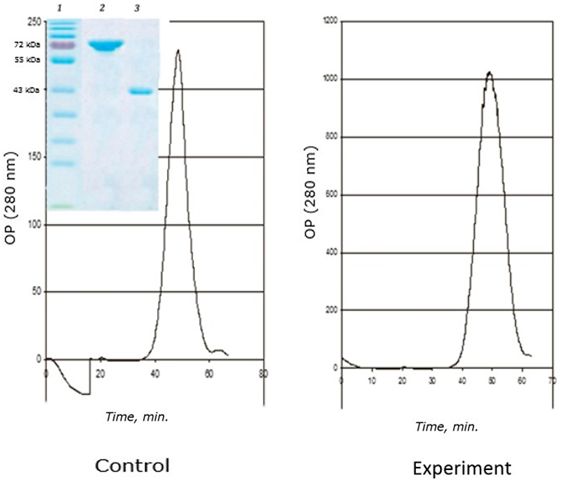
Fig. 8. Chromatographic profile (FPLC, Superosa 12, λ 280 nm) of the resuspended acetone residue (fraction D) of the digestive gland of 1-year-old scallops M. yessoensis (control and experiment – CdCl2 300 μg/l) and SDS-electrophoresis (9 %) of the eluted peaks (1 and 2). Coomassie blue stained
A feature of this age is also the amount of cadmium bound to the protein 72 kDa. In control individuals of M. yessoensis, this protein binds 2.08 μg of Cd/mg of protein, which is 20 times higher than in yearlings and 11.5 times higher than in two-year-olds (Fig. 9).
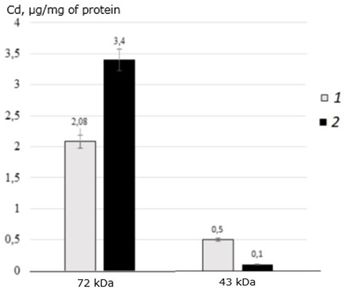
Fig. 9. The distribution of cadmium (μg/mg of protein) among the MT-like proteins of the digestive gland of three-year-old scallops M. yessoensis in control and experimental groups (CdCl2 300 μg/l)
Antioxidant activity identified cadmium-binding proteins have a three-year-old individuals of scallop M. yessoensis is significantly different from cadmium-binding proteins in yearlings and differs slightly from the two-year-old individuals. Thus, the value of the AO activity of the 72 kDa protein is 1.7 times lower in three-year-olds in both the control group and the experimental group compared to one-year-olds. Compared with two-year-olds, the value of AO protein activity of 72 kDa increased from 42 to 65.33 mMol of Trolox/mg of protein for the control and from 42 to 72 mMol of Trolox/mg of protein for the experiment.
The results also showed (Fig. 10) that in the natural habitat, the cadmium-binding protein 43 kDa has AO activity – 110.28 mMol of Trolox/mg of protein, which increases sharply to 153.75 mMol of Trolox/mg of protein during incubation of scallops with cadmium, which is the highest indicator of AO activity among the cadmium-binding proteins of all three studied groups. It is seen that the main cadmium-binding protein 72 kDa does not change the AO activity. The 43 kDa protein in three-year-olds has mainly the AO function in high cadmium content in the environment.
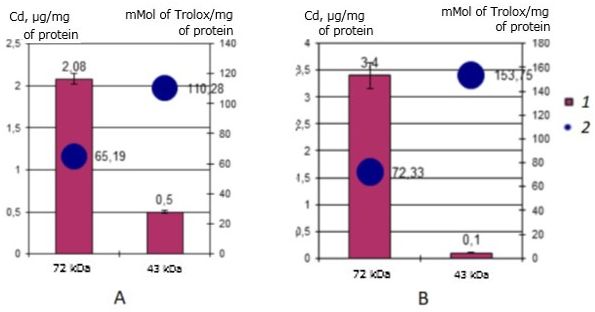
Fig. 10. AO activity (mM Trolox/mg protein) of MT-like proteins of the digestive gland in three-year-old scallops M. yessoensis in control (A) and experimental (B) groups
Discussion
In the course of the experiment, it was found that the largest amount of cytoplasmic cadmium is observed in the age group of yearlings, a smaller amount – in three-year-olds, followed by two-year-old individuals of M. yessoensis. This result is typical for both control and experimental mollusks. Thus, the results of earlier studies (Бельчева, 2002) on the accumulation of cadmium in the soft tissues of the seaside scallop need to be supplemented. Since the authors did not conduct studies on one-year-olds and there is no data on accumulation in two-year-olds, we can say that in the age group of 1-3 years, the concentration of cadmium in the digestive gland does not depend on the size of the shell, at least for cage scallops. So, during the experiment, it was found that the scallops of the 2nd year accumulate cadmium in a smaller amount. We assume that this fact is due to the fact that in the cage conditions of the maricultural industry, the seaside scallop reaches sexual maturity in two years, so in this age group there is a restructuring of metabolic pathways and this age is not indicative when studying the accumulation of cadmium. This fact is confirmed both in the tissue accumulation of metals (Cd, Zn and Cu), where metals are least accumulated, and in the study of the distribution of cadmium at the stage of subcellular fractionation, where the share of cytoplasmic cadmium accounts for only 10% in the control and 20% in the experiment. In studies of the accumulation of metals in the digestive gland of M. yessoensis (Силина, 2004), no cadmium was found in two-year-old individuals of a scallop selected in June from the Amur Bay area (severe anthropogenic pollution), and in three-year-olds, cadmium reached 142 μg/g dry tissue.
The distribution and level of accumulation of physiologically important metals in the digestive glands of the scallop depend on the season and age (Бельчева, 2002). The greatest accumulation of zinc metals in the digestive gland is observed in March with an increase in the weight of the digestive gland, and copper – in June. However, the authors did not consider the age of the scallop less than 3 years. The accumulation of cadmium does not depend on the season and correlates with the shell size. This statement can be considered true if the background concentrations of cadmium in the environment are met. In our experiment conducted in June, we can see that with increasing shell size and, as a result, age, the amount of accumulated cadmium decreases.
The study of age-related features of accumulation of such metals as cadmium, zinc and copper in the scallop was conducted earlier (Lukyanova et al., 1993). It is generally accepted that as the mass of soft tissues increases, more metals accumulate in different members of the Pectinidae family (Greig et al., 1978; Mauri et al., 1990; Uthe, Chou, 1987). However, our study states that under conditions of high cadmium content in the environment, the degree of accumulation of this metal in the age group of 1-3 years does not depend on the shell size. However, the accumulation of cadmium in the digestive gland depends on age. No similar studies have been found on other species of the family Pectinidae that are 1 year old. We noted that yearlings of the scallop accumulate more cadmium under heavy metal load conditions due to the presence of a high-molecular-weight cadmium-binding MT-like protein in the digestive gland tissue. It was also found that starting from three-years-old, metabolic processes are stabilized and the main role in the binding of cadmium belongs to a protein with a molecular weight of 72 kDa. However, in studies on 5-7-year-olds, it was found that under control conditions, the main cadmium-binding protein is 48 kDa, and under conditions of cadmium loading – 72 kDa (Zhukovskaya et al., 2012).
Our study also identified and evaluated the ability of Cd-binding MT-like proteins to exhibit antioxidant properties in the seaside scallop in response to the ingestion of cadmium. It is known that in response to the action of prooxidants, the body reacts with a cascade of reactions of the antioxidant system – an antioxidant response. One of the variants of the body antioxidant protection in response to stress of different nature the level of MT increases in the body of marine invertebrates (Viarengo et al., 1988; Zapata-Vivenes et al., 2007). Therefore, these proteins are often used by researchers as biomarkers of specific stress. In our work, the study of the MT-like proteins general anti-radical activity in the digestive gland of the seaside scallop M. yessoensis showed that the studied proteins are able to absorb oxygen radicals, and therefore are one of the mechanisms of antioxidant protection in the seaside scallop. The decrease in the level of TOSC in the studied metal-binding proteins in the immature scallop can be considered as a mechanism of adaptation to changing environmental conditions. These results are correlated with results on other representatives of bivalves. For example, similar studies were performed on the Mytilus galloprovincialis mussel (Viarengo et al., 2000), where the authors showed that accumulated cadmium binds with MT, which at a non-toxic dose of cadmium have antioxidant properties.
Conclusions
As a result of the experiment, we were able to detect age-related features in the accumulation of cadmium in the seaside scallop M. yessoensis. It was found that one-year-old individuals of the seaside scallop are able to bind cadmium in greater quantities among all studied ages (1, 2 and 3 years). This feature is consistent with the presence of a protein with a molecular weight of 120 kDa in the tissue of the digestive gland. It was found that this protein loses the binding cadmium function in two-year-olds and is not synthesized in three-year-old scallops in the digestive gland at all, either in control or in response to high concentrations of cadmium in the environment. Two-year-old individuals of the scallop do not bind cadmium in quantities similar in value with three-year-old and one-year-old individuals of the scallop, in connection with the restructuring of the biochemical device and transfer the shellfish to mature stage. Three-year-olds stabilize their biochemical apparatus of adaptation to cadmium, and their ability to bind cadmium is comparable to adults, 5-7 years old, individuals of M. yessoensis.
References
Bartosz J. A., Ertel D., Bartosz M. Simple determination of peroxyl radical-trapping capacity, Biochemistry and molecular biology international. 1998. Vol. 46. No 3. P. 519–528. DOI: 10.1080/15216549800204042.
Bel'cheva N. N. Silina A. V. Slin'ko E. N. Chelomin V. P. Seasonal variability of the levels of Fe, Zn, Cu, Mn and Cd in the hepatopancreas of the scallop Mizuhopecten yessoensis, Biologiya morya. 2002. T. 28. No. 6. P. 442–448.
Chelomin V. P., Belcheva N. N. Alterations of microsomal lipid synthesis in gill cells of bivalve mollusc Mizuhopecten yessoensis in response to cadmium accumulation, Comp. Biochem. and Physiol. 1991. Vol. 99 C. No. 1–2. P. 1–5.
Chelomin V. P., Bobkova E. A., Lukyanova O. N., Chekmasova N. M. Cadmium-induced alterations in essential trace element homeostasis in the tissues of scallop Mizuhopecten yessoensis, Comp. Biochem. and Physiol. 1995. Vol. 110 C. No. 3. P. 329–335.
Fowler B. A., Gould E. Ultrastructural and biochemical studies of intracellular metal-binding patterns in kidney tubule cells of the scallop, Placopecten magellanicus, following prolonged exposure to cadmium or copper, Mar. Biol. 1988. Vol. 97. P. 207–216.
Greig R. A., Wenzloff D. R., MacKenzie C. L. Jr., Merrill A. S., Zdanowicz V. S. Trace metals in sea scallops, Placopecten magellanicus, from eastern United States, Bull. Environ. Contam. Toxicol. 1978. Vol. 19. P. 326–334.
Laemly K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4, Nature. 1970. No. 5259. P. 680–685.
Lowry O. H., Rosebrough N. J., Farr A. L., Randall R. J. Protein measurement with the Folin phenol reagent, Journal of Biological Chemistry. 1951. Vol. 193. P. 265–275.
Lukyanova O. N., Belcheva N. N., Chelomin V. P. Cadmium bioaccumulation in the scallop Mizuhopecten yessoensis from an unpolluted environment, Ecotoxicology of metals in invertebrates. Edited by R. Dalinger and F. Rainbow. Lewis Publisher, 1993. P. 25–35.
Mauri M., Orlando E., Nigro M., Regoli F. Heavy metals in the antarctic scallop Adamussium colbecki, Mar. Ecol. Prog. Ser. 1990. Vol. 67. P. 27–33.
Ponzano E., Dondero E., Bouquegneau J, M., Sack R., Hunziker P., Viarengo A. Purification and biochemical characterization of cadmium metallothionein from the digestive gland of the Antarctic scallop Adamussium colbecki (Smith, 1902), Polar Biology. 2001. Vol. 24. P. 147–153.
Roesijadi G., Fowler B. A. Purification of invertebrate metallothioneins, Methods in Enzymology. 1991. Vol. 205. P. 263–273.
Roesijadi G., Kielland S. L., Klerks P. Purification and properties of novel molluscan metallothioneins, Archiv. Biochem. Biophys. 1989. Vol. 273. P. 403–413.
Silina A. V. Bel'cheva N. N. Age and seasonal variability of concentrations of physiologically important metals in the digestive gland of seaside scallop from polluted and clean areas, Byulleten' Dal'nevostochnogo malakologicheskogo obschestva. 2004. Vyp. 8. P. 56–67.
Stone H. C., Wilson S. B., Overnell J. Cd-binding proteins in the scallop Pecten maximus, Environ. Health Perspect. 1986. Vol. 65. P. 189–191.
Stone H. C., Wison S. B., Overnell J. Cadmium binding components of scallop (Pecten maximus) digestive gland. Partial purification and characterization, Comp. Biochem. Physiol. 1986. Vol. 85 C. P. 259–268.
Thompson J. A. J., Sutherland A. E. A. Comparison of methods for sample clean-up prior to quantification of metal-binding proteins, Comp. Biochem. Physiol. 1992. Vol. 102. No 4 B. P. 769–772. DOI: 1016/0305-0491(92)90077-5.
Uthe J. F., Chou C. L. Cadmium in sea scallop (Placopecten magellanicus) tissues from clean and contaminated areas, Canad. J. Fish. Aquat. Sci. 1987. Vol. 44. P. 91–98.
Viarengo A., Burlando B., Certto N., Panfoli I. Antioxidant role of metallothioneins: a comparative overview, Cell. Mol. Biol. 2000. Vol. 304. No. 46 (2). P. 407–117.
Viarengo A., Pertica M., Canesi L., Biasi F., Cecchini G., Orunesu M. Effects of heavy metals on lipid peroxidation in mussel tissues, Mar. Environ. Res. 1988. Vol. 24. P. 355–359.
Zapata-Vivenes E., Nusetti O. Protection of glycolytic enzymes by metallothioneins from oxidative damage in the digestive gland of green lipped mussel Perna viridis, Journal of Shellfish research. 2007. Vol. 26. No. 2. P. 335–344.
Zhukovskaya A. F., Belcheva N. N., Slobodskova V. V., Chelomin V. P. Metallothionein-like proteins induced by cadmium stress in the scallop Mizuhopecten yessoensis, Ocean Science Journal. 2012. Vol. 47. No. 3. P. 189–195.





 © 2011 - 2025
© 2011 - 2025