Introduction
The water vole (Arvicola amphibius) inhabits different landscapes, including anthropogenic ones and has great biocenotic and epidemiological significance (Водяная полевка, 2001; Проскурняк, Назарова, 2017; Steward et al., 2017). Demographical structure of the population is closely connected with the reproductive intensity and mortality rate depending on climatic factors and landscape-ecological features of the habitat (Рогов, 1999; Рогов и др., 1999; Potapov et al., 2004; Проскурняк, Назарова, 2017). At present, the age composition of the adult rodent population is insufficiently investigated, and ecologo-phisiological features of senile individuals and their role in the population life, in the spread of infections remain unclear, which is due to the lack of reliable signs and criteria for determining age. By the data of individual marking and recaptures conducted in Severnaya Baraba, it was stated that about 7% of individuals live less than 2 years (Пантелеев, 1966; Водяная полевка, 2001). In the long-term population investigation conducted in France it was estimated that maximum life span of water voles exceeds 2 years in nature (Saucy, 1988). In literature there is an evidence that individuals older than 2 years occur in the Microtus duodecimcostatus. Other representatives of the family: Microtus agrestis, Microtus arvalis, Microtus pennsylvanicus, Microtus townsendii, Microtus californicus, Microtus montanus, Myodes glareolus have maximal life span not more than 20 months (Paradis, Guerdon, 1993). We propose that long-lived individuals play a significant role in maintaining the number and genetic diversity of water vole populations (Назарова, Проскурняк, 2017). In captivity water voles live up to 3.5 years, preserving the ability to reproduce throughout life (Назарова, 2011). Ecology-evolutionary value of long-lived individuals can significantly increase in pessimal ecological conditions, when participation in reproduction of young individuals is limited. The reduced contribution of this-year brood rodents in reproduction is typical for the decline in numbers, when the duration of the reproductive period is reduced and the puberty of young animals is inhibited (Водяная полевка, 2001). The participation in reproduction for this-year brood is also limited in the populations inhabiting anthropogenic landscapes (Проскурняк, Назарова, 2017) or living in the area periphery (Музыка, 1988) due to the lack of suitable breeding areas.
Previously, we developed a method for determining the age of water voles with an accuracy of up to a year, by measuring the bones of the pelvic limbs using discriminant analysis (Zudova et al., 2017). However, this method does not take into consideration calendar dates of death of an animal, which can influence the precision of age determination, since the water voles seasonal rhythms of growth – its spring acceleration and autumn regression – are clearly pronounced (Водяная полевка, 2001).
The goals of this study: 1) to develop a method for determining the age of water voles by morphometric characteristics of the pelvic extremities, taking into account the calendar dates for collecting of field material (late spring – early autumn); 2) to find out the age composition of overwintered individuals in two populations of water voles that live on garden plots.
A detailed study of the demographic composition of the adult rodent population is important for determining the mechanisms of maintaining the viability of populations and the evolution of longevity.
Materials
The material for the study was collected at two sites in the Novosibirsk region. In August – September 2017, trapping was carried out on the territory of the gardening partnership "Sibselmashevets", located 1 km from the Oyosh railway station (55º03'58" N. lat., 81º47'05" E. lon.). The place of capture is a steppe meadow located between the swampy birch-aspen groves. A total of 14 wintering voles (5 males and 9 females) and 19 yearlings (9 males and 10 females).
Near the Novosibirsk scientific center (NSC – Akademgorodok), animals were caught in April-September 2014-2018 on the territory of the garden partnership "Nadezhda-2 "(54º49 ' 17 "s. sh., 83º09'48" V. d.), 100 meters from the aspen-birch forest. In ravines and depressions there are silted ponds connected by streams. A total of 22 overwintered individuals (14 males and 8 females) and 31 youngsters (19 males and 12 females) were caught here. The maximum number of animals, 30 individuals, was captured in 2018. Wintering individuals were captured mainly in the spring (April-May). After July 10, they did not meet in the catches.
The biological feature of garden plots is that the water vole breeds near reservoirs, and uses the gardens as forage and wintering stations. To the nearest water bodies in the NSC there is about 1.5 km, in Oyosh – from 500 m to 1 km.
To develop a method of age determination, the water voles of the vivarium breeding (n = 139) and water voles caught in 2014-2017 in the area of the vil. Licyi Norki of Ubinsky district of Novosibirsk region (55º52' 09" N., 80º05'03" E.) and placed in the vivarium, where they were held until the end of their life (n = 14), were used. The choice of capture location is explained by the fact that the laboratory colony is founded by individuals just from this population.
In the vivarium, water voles were kept in individual cages, provided with hay, in conditions of natural light period, free access to water and food (grain mix, carrots, oat sprouts).
Methods
In Oyosh, water voles were caught in cylinders (35 x 15 cm) dug along vegetable beds. The distance between the cylinders averaged about 3 m. To obtain information on the long-term population dynamics, the widely spread in environmental studies method was used (Максимов, 1967; Методические указания..., 1974; Карасева, Телицина, 1996). We interviewed only those owners of garden plots who in 2014-2018 caught the pest themselves and knew this species well. Respondents estimated the number of animals in conventional units: 5 – very much; 4 - many; 3 - little; 2 - single; 1 - no (Проскурняк, Назарова, 2017). In total 11 people was interviewed.
Near Akademgorodok, voles were caught using a groove (50 meters with 5 cylinders), which was located along the borders of the garden plot, near the borders of the partnership.
As additional information confirming the nature of the populational change, we used the intensity of reproduction of increment animals (Рогов, 1999; Cerqueira et al., 2006). Breeding females included individuals with embryos or placental spots in the uterus. To males capable of reproduction – individuals who had mature spermatozoa in the appendages of the testes.
To the overwintered, the voles were included, in which the length of the common medial crest on the skull, formed by the merged longitudinal sagittal crests, exceeded or was equal to 3 mm (Пантелеев, 1966). As an additional criterion, the general structure of the skull was taken into account: the roof of the skull of old voles "seems to be sunk among the edging ridges" (Водяная полевка, 2001).
To divide overwintered animals into age classes (those who survived one winter; more than two winters) by the dimensions of pelvic limb bones, the discriminant analysis was used. To find discriminant functions, a control sample was used (42 females and 41 males), represented by voles of a known age, that was born in vivarium and died in the same months as those captured in nature (in April – September). All of them have lived in the vivarium for at least one year.
Since there is sexual dimorphism in skeletal features (Somoano et al., 2017; Zubova et al., 2017), discriminant analysis was performed separately for males and females using measurements:
- a) Pelvic bone. Isl is the length of the ischium, measured from the edge of the acetabulum to the ischial tuberosity; ISW – maximum width of the body of the ischium; PBL – the greatest long pubis, measured from the edge of the acetabulum; PH – height of the pelvis along a line directed perpendicularly from the ischial tuberosity to the line length of the pubis, measured on the outer edge; OFW – the width of the obturator foramen.
- (b) Femur, dorsal plane. CFW – width of the femoral neck; FLp – length of the proximal part of the femur, including the third trochanter; FWp – width of proximal part of femur at the third trochanter; FWd – distal width of the femur; FWCd – the width of the distal part of the femur, measured at the greatest protrusions of the lateral and medial epicondyle.
- c) Femur, lateral plane. FWpl – width of the proximal part of the femur; FWdl-width of the distal part of the femur.
- (d) Crus. TWp – width of the proximal part of the crus.
Each measurement was performed using an electronic caliper (accuracy 0.01 mm) on the left pelvic limb in three repetitions, then the average was calculated.
The equations of the classification function had the form:
y = Cl + kl · xl + ... + kn · xn,
where Cl is a constant, k1-n is the coefficients of the classification of the function of the -- feature, and xl is the value of the feature for the certain exemplar.
To verify the accuracy of determining age using classification functions, a test sample of 29 males and 27 females born in vivarium was used. The test and control samples were balanced by the proportion of animals of different ages (Клевзаль и др., 2005). Individuals of the test sample were not included in the control sample. In addition, the correctness of the age determination was checked on another sample – 7 males and 7 females caught in a population living in the vicinity of the vil. Lisyi Norki in the Novosibirsk region, and kept in a cage for the rest of their lives in vivarium. The relative age of the animals in this group was examined during the capture (wintering, fledglings). Age was determined by the calendar terms of capture, body size, state of the coat, and reproductive status (Водяная полевка, 2001; Карасева и др., 2008).
To determine the age of individuals in the test samples, taking into account their gender (1 year, 2 years or more), the values of two classification functions were calculated using coefficients calculated separately for males and females. The observation was assigned to the group for which the classification function had the highest value. The same algorithm was used to determine the age of water voles from natural populations.
To determine the relationship with the age of craniometric characteristics, the following measurements were taken:
CBL – condylobasal length of skull – distance from most forward point of the maxillary bones to the furthest back point of the occipital condyles; CHTB – height of the skull from the drum cameras – distance from the lowest point of the drum of both cameras to the highest point of the skull roof; ICL – is the length of the fused part of the interorbital crests; NL – length of nasal bones – the distance between the most protruding forward and backward points of the nasal bones; NW – the width of the nasal bones – the distance between the most protruding points of the nasal bones; UMS – length of the upper row of molars – from the posterior edge of the alveoli of the molars to the anterior edge of the alveoli of the first molars; ZW – zygomatic width of the skull – the greatest distance between the outer edges of the zygomatic arches.
Statistical data processing was performed using the Statistica 6.1 software package (SAS Institute, USA). The distribution of morphometric skeletal features corresponded to the law of normal distribution. Homogeneity of variance in different groups was evaluated by the Leven criterion, and no statistically significant differences were found. To find the coefficients of linear classification functions, we used step-by-step discriminant analysis with sequential inclusion of variables. To determine the effect of specific factors on variability morphometric features were used one-and two-factor analysis of variance. Multiple comparisons of averages were performed using the Scheffe criterion, and paired comparisons were performed using the Student's t-criterion. Differences between shares were evaluated using the Chi-square method. The text and tables show the average values of features (X), standard error (± SE), and sample size (n). The level of statistical significance is assumed to be p < 0.05.
Results
Numbers
Fig. 1 shows the average annual dynamics of the number of water voles that live in areas. In 2016-2018, the number of Oyoshin population decreased, and the population from Akademgorodok increased.
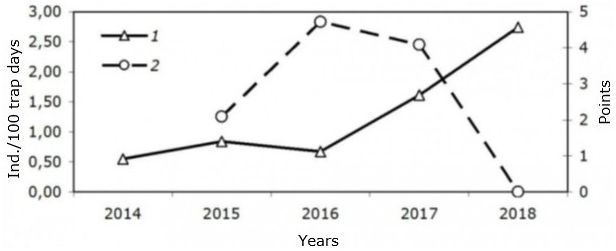
Fig. 1. The average annual dynamics of the number of two populations of water voles. 1 – Academgorodok, 2 – Oyosh. Оyosh – scoring; Akademgorodok - ind./100 trap days
The body size of the individuals which wintered
In the literature, there is no information about the exterior characteristics of water voles that live on garden plots. Data on the weight, body length and fatness (the ratio of body weight to length) of wintering individuals of the studied populations are given in table. 1. As shown by the results of two-factor analysis of variance, body weight depends on the place of capture (F1.32 = 17.30, p < 0.001) and sex of animals (F1.32 = 5.56, p < 0.05), and body length and fatness – only on the place of capture (F1. 32 = 35.67, p < 0.001 and F1. 32 = 4.39, p < 0.05, respectively). However, the results of comparison of group averages using the Scheffe criterion showed that there are no significant differences between animals of the same sex, but different places of capture.
Table 1. Body size of wintering individuals from two populations living on garden plots
| Capture place | Sex | Body weight, g | Body length, mm | Fatness | n |
| Akademgorodok | female | 131.1 ± 4.3 | 173.5 ± 1.9 | 0.76 ± 0.04 | 8 |
| male | 143.0 ± 4.5 | 173.7 ± 2.4 | 0.82 ± 0.03 | 14 | |
| Oyosh | female | 156.4 ± 10.2 | 188.3 ± 2.6 | 0.83 ± 0.04 | 9 |
| male | 179.3 ± 11.5 | 196.4 ± 5.7 | 0.91 ± 0.05 | 5 |
Table. 2 shows the weight, body length and fatness of animals bred in the vivarium to compare the exterior characteristics of wild and vivarium individuals and to analyze the age-related changes. The results show that after reaching adulthood, water voles continue to grow. The effect of age (1, 2, or 3 years) on body weight and, especially, body length is statistically significant. Females: body weight – F2,68 =3.93, p < 0.05, body length – F2,68 = 22.02, p < 0.001, fatness – F2,68 =1.89, p > 0.05. Males: body weight – F2,67 = 6.48, p < 0.01, body length – F2,67 = 98.73, p < 0.001, fatness – F2,67 = 5.46, p < 0.05. Therefore, postcranial skeleton measurements can be used to determine the age of adult animals.
Table 2. Exterior characteristics of the vivarium water voles at different ages
| Sex | Age, years | Body weight, g | Body length, mm | Fatness | n |
| Female | 1 | 143.2 ± 5.3 | 177.4 ± 1.6 | 0.80 ± 0.02 | 37 |
| 2 | 169.2 ± 9.4 | 188.8 ± 2.0 | 0.89 ± 0.04 | 24 | |
| 3 | 168.5 ±13.2 | 197.7 ± 2.3 | 0.85 ± 0.06 | 10 | |
| Male | 1 | 180.0 ± 5.8 | 185.3 ± 1.5 | 0.97 ± 0.02 | 36 |
| 2 | 219.6 ± 9.7 | 197.8 ± 2.0 | 1.10 ± 0.04 | 27 | |
| 3 | 186.0 ± 22.4 | 205.7 ± 4.7 | 0.89 ± 0.09 | 7 |
Discriminant functions for determining the age of males and females
The results of the discriminant analysis performed on the control sample were highly reliable: females – λ = 0.16, F8.32 = 20.55, p < 0.001; males – λ = 0.15, F10.30 = 17.06, p < 0.001. The coefficients of the linear classification function are given in table. 3.
Table 3. Coefficients of linear classification functions for age determination
| Characters | Females | Males | ||
| age, years | age, years | |||
| 1 | ≥ 2 | 1 | ≥ 2 | |
| Pelvic bone | ||||
| ISW | -3.867 | -5.661 | 45.232 | 56.180 |
| PH | -10.766 | -15.557 | ||
| PBL | 20.047 | 23.750 | ||
| Isl | 5.544 | 8.904 | 23.993 | 28.571 |
| OFW | 65.906 | 73.252 | ||
| Femur | ||||
| FLp | 29.413 | 33.426 | ||
| CFW | 1.948 | -16.875 | 187.312 | 201.959 |
| FWp | -82.320 | -96.654 | ||
| FWpl | 28.325 | 52.741 | -81.488 | -67.683 |
| FWd | -5.827 | -10.556 | 64.477 | 79.721 |
| FWdl | 2.124 | -17.036 | ||
| FWCd | 144.265 | 151.284 | ||
| Shin | ||||
| TWp | -35.424 | -47.156 | ||
| Constant | -339.303 | -444.253 | -745.375 | -878.396 |
| Correctness of definition, % | 100 | 100 | 100 | 100 |
The accuracy of classification of individuals in the control sample is 100 %. The accuracy of classification of individuals in the test sample is 89.7 % for males and 81.5 % for females. The accuracy of classification of animals caught in nature and those that died in vivarium is 100 % for females and 71.4 % for males.
The results of determining the age of overwintered water voles from two natural populations that live on garden plots are presented in table. 4.
Table 4. The age composition of the overwintered water voles
| Age, years | Akademgorodok | Oyosh | ||
| females | males | females | males | |
| 1 | 8 | 10 | 7 | 3 |
| ≥ 2 | 0 | 4 | 2 | 2 |
| % that survived more than 2 winters | 0 | 28.6 ± 12.1 | 22.2 ± 13.8 | 40.0 ± 21.9 |
In general, in the Oyosh population the overwintered individuals that lived for more than two winters amounts 33.3%, and in the population from the vicinity of Akademgorodok – 18.8%. In both populations, the proportion of long-lived individuals in females is lower than in males. Summing up data for two populations, one can see that 11.8 % of females and 31.6 % of overwintered males are more than 2 years old. Differences in the age composition of males and females are statistically insignificant (χ2 = 2.04, p > 0.05).
Craniometric characteristics of wintering individuals of different age classes
Males of different ages (groups: 1 year, 2 years and older) from the population of Akademgorodok significantly differed in craniometric characteristics – the width of the nasal bones and the height of the skull (table. 5), the features that have a strong correlation with age (Назарова и др., 2015). There were no significant gender and age differences in craniometric characteristics in the Oyosh population.
Table 5. Craniometric characteristics of males of different ages
| Characters | Age | P | |
| 1 year | 2 years and older | ||
| Number of animals | 10 | 4 | |
| Nasal bones width | 4.32 ± 0.05 | 4.55 ± 0.07 | < 0.05 |
| Skull height | 12.57 ± 0.14 | 13.13 ± 0.22 | 0.05 |
Participation in reproduction
The reproductive status of juveniles caught in the vicinity of Akademgorodok was assessed in 25 individuals. Of these, 40 % of males were sexually mature (had mature spermatozoa in the appendages of the testes), and 50 % of females (n = 10) participated in reproduction (had embryos or placental spots in the uterus), which indicates a high reproductive potential of this population, which is in the phase of population growth.
In the Oyosh population, there were no sexually mature males (n = 9) and only 30 % of young females (n = 10) took part in breeding. A similar level of reproductive activity is typical for populations in the decline – depression phase (Водяная полевка, 2001).
In the group of overwintered females, reproductive indicators were evaluated in 9 females, 5 males from the Oyosh population, and 7 females and 13 males from the Akademgorodok population. All wintering females took part in breeding.
In the Oyosh population, the total weight of testes in two-year-old males was significantly higher than in one-year-olds: 2.09 ± 0.01 and 0.36 ± 0.14 g, respectively (F1.3 = 95.46, p < 0.01). In one-year-old males, the testes showed signs of regression associated with the end of the breeding season (Проскурняк, Назаров, 2017). In the population of Akademgorodok, age-dependent differences in the size of testes were not found in overwintered males.
Discussion
The method we developed for determining the age of water voles based on measurements of pelvic limb bones was used to assess the age structure of two populations. It was found that on average about 25 % of individuals survive two or more winters. There is a tendency to increase the proportion of twice-wintering animals in the population with decreasing numbers, which confirms the predictions of the Boonstra aging hypothesis (Boonstra, 1994). According to this hypothesis, the increase in the age of animals on the decline in population is associated with inhibition of puberty of youngsters and shortening of the breeding season. In turn, increasing the age of breeding animals may be associated with a decrease in homeostasis of physiological functions, weakening of resistance to stress, reproductive aging and population decline. Other authors have also noted an increase in the proportion of older animals in the population during the decline in the water vole population (Cerqueira et al., 2006).
Inter-population differences in the exterior characteristics of water voles inhabiting garden plots were found. Individuals from the Oyosh population are larger than those from the Akademgorodok population, that may be due to the antiphase dynamics of the number of compared populations and differences in the age structure of populations (Рогов, 1999; Рогов и др., 1999).
The results of earlier studies of age-related changes in the reproductive parameters of water voles in vivarium conditions showed that females of the second year of life have a higher probability of estrus and mating when pairing with a male than females of the first and the third year of life. Signs of reproductive aging in water voles appear only in the third year of life (Назарова, Проскурняк, 2017). In studies on other mammals, it was found that the success of reproduction has a quadratic dependence on age. Middle-aged individuals have better reproductive qualities (Beauplet et al., 2006).
Olfactory signals play an important role in age-dependent realization of reproductive potential. In Microtus pennsylvanicus, the olfactory signals of older males are more attractive to females than the olfactory signals of sexually mature young males, while long-lived males are more interested in the olfactory stimuli of females than young ones (Ferkin, 1999). We suggest that during the peak and decline phases, when breeding territories are reduced and male competition for access to females increases, long-lived individuals may have a selective advantage. In population studies conducted in Northern Baraba, it is shown that during the downturn, larger males locate themselves closer to reproductively active females (Водяная полевка, 2001). Water voles, according to the results of the study, grow throughout their lives, so the reproductive success of males may increase with age. In two-year-old males of the Oyosh population, wintering on garden plots (Проскурняк, Назарова, 2017), the mass of testes is significantly higher than in one-year-olds. Two-year-old males living near Akademgorodok are superior to one-year-olds in some craniometric characteristics.
Conclusions
The age composition of overwintered water voles in two populations that differ in population dynamics was established. There is a tendency to increase the age of overwintered animals caught in a population that is in the period of population decline, which is consistent with prediction of the hypothesis of R. Boonstra about the aging of the population during the recession. Long-lived individuals play an important role in maintaining the viability of populations in pessimal environmental conditions. The results are important for understanding the ecological mechanisms of longevity evolution and monitoring the status of water vole populations in anthropogenic landscapes.
References
Beauplet G., Barbraud C., Dabin W., Kűssener C., Guinet C. Age-specific survival and reproductive performances in fur seals: evidence of senescence and individual quality, Oikos. 2006. Vol. 112. P. 430–441.
Boonstra R. Population hypothesis cycles in microtines: the senescence hypothesis, Evolutionary Ecology. Vol. 8. P. 196–219.
Cerqueira D., de Sousa B., Gabrion C., Giraudoux P., Quéré J. P., Delattre P. Cyclic changes in the population structure and reproductive pattern of the water vole, Arvicola terrestris Linnaeus, 1758, Mamm. Biol. 2006. Vol. 71. P. 193–202.
Ferkin M. H. Attractiveness of opposite-sex odor and responses to it vary with age and sex in meadow voles (Microtus pennsylvanicus), Journal of Chemical Ecology. 1999. Vol. 25. No 4. P. 757–769.
Gelling M., Macdonald D. W., Telfer S., Jones T., Bown K., Birtles R., Mathews F. Parasites and pathogens in wild populations of water voles (Arvicola amphibius) in the UK, European Journal of Wildlife Research. 2012. Vol. 58. No 3. P. 615–619.
Karaseva E. V. Telicyna A. Yu. Zhigal'skiy O. A. Methods of studying rodents in the field. M.: Izd-vo LKI, 2008. 416 p.
Karaseva E. V. Telicyna A. Yu. Methods of studying rodents in the field. M.: Nauka, 1996. 227 p.
Klevezal' G. A. Mina M. V. Krushinskaya N. L. Using the methods of multidimentional statistical analysis in determining the age of mammals (on the example of the forest mouse, Sicista betulina, and the pine marten, Martes martes), Zoologicheskiy zhurnal. 2005. T. 84. No. 11. P. 1389–1401.
Maksimov A. A. Water rat hazard zone in Western Siberia, censuring methods and forecast. Novosibirsk: Nauka, 1967. 58 p.
Methodological guidelines on accounting the number and prediction of outbreaks of mass breeding of water rats in Western Siberia, Minsel'hoz RSFSR. Gl. upr. zaschity rasteniy. BI SO RAN. Kuybyshev (NSO): P–o Prostor., 1974. 29 p.
Muzyka V. Yu. The structure of a local water vole population, Ekologiya populyaciy. Ch. 1: Tezisy dokladov Vsesoyuznogo soveschaniya (4–6 oktyabrya 1988 g., Novosibirsk). Novosibirsk, 1988. P. 53–55.
Nazarova G. G. Proskurnyak L. P. Age-dependent variability of reproductive parameters in the water vole (Arvicola amphibius, 1758), Vestnik IrGSHA. 2017. No. 83. P. 141–145.
Nazarova G. G. Zudova G. A. Proskurnyak L. P. Age-dependent variability and sexual dimorphism of craniometrical characters in water vole (Arvicola amphibius, Rodentia, Arvicolinae), Zoologicheskiy zhurnal. T. 94. No. 8. P. 955–962.
Nazarova G. G. Effects of seasonal, ontogenetic, and genetic factors on lifespan of male and female progeny of Arvicola amphibius, Frontiers in genetics. T. 4. No. JUN. P. Article 100.
Panteleev P. A. Experience in determining the age of water voles in the autumn population, Byull. MOIP. Otd. Biol. 1966. T. 71. Vyp. 4. P. 20–25.
Paradis E., Guedon G. Long lifespan in a population of Microtus (Pitymys) duodecimcostatus, Mammalia, De Gruyter. 1993. Vol. 57. No 1. P. 142–144.
Potapov M. A., Rogov V. G., Ovchinnikova L. E., Muzyka V. Yu., Potapova O. F., Bragin A. V., Evsikov V. I. The effect of winter food stores on body mass and winter survival of water voles, Arvicola terrestris, in Western Siberia: the implications for population dynamics, Folia Zoologica. 2004. T. 53. No. 1. P. 37–46.
Proskurnyak L. P. Nazarova G. G. Population, population structure and harmful activity of the water vole (Arvicola amphibius) inhabiting garden plots, Pest-Menedzhment. 2017. No. 4 (104). P. 23–29.
Rogov V. G. Potapov M. A. Evsikov V. I. Gender structure of populations of the water vole Arvicola terrestris (Rodentia, Cricetidae) in Western Siberia, Zoologicheskiy zhurnal. 1999. T. 78. No. 8. P. 979–986.
Rogov V. G. Population dynamics and demographic parameters of the water vole (Arvicola terrestris L.) population in the subtaiga zone of the Western. Novosibirsk: ISiEZh SO RAN, 1999. 19 p.
Saucy F. Dynamique de population, dispersion et organisation sociale de la forme fouisseuse du campagnol terrestre, (Arvicola terrestris scherman (Shaw), Mammalia, Rodentia). These Universite de Neuchatel. 1988. 366 p.
Somoano A., Ventura J., Miñarro M. Continuous breeding of fossorial water voles innorthwestern Spain: potential impact on apple orchards, Folia Zool. 2017. Vol. 66. No. 1. P. 37–49.
Stewart R. A., Clark T. J., Shelton J., Stringfellow M., Scott C., White S. A., McCafferty, D. J. Urban grasslands support threatened water voles, Journal of Urban Ecology. 2017. No 3. P. 1–7.
Water Vole: Species Image. M.: Nauka, 2001. 527 p. (Seriya «Vidy fauny Rossii i sopredel'nyh territoriy).
Zudova G. A., Proskurnyak L. P., Nazarova G. G. Age and sex determination in the water vole (Arvicola amphibius, Rodentia, Arvicolinae) based on measurements of the pelvic limb bones, Biology Bulletin. 2017. Vol. 44. No 9. P. 1115–1122.





 © 2011 - 2026
© 2011 - 2026