Introduction
Polymorthic freshwater fish of the Northern hemisphere that colonized reservoirs of deglacified areas in the post-glacial period are widely studied models of allopatric and sympatric divergences as niche adaptations (Schluter, 1996; Bernatchez, 2004; Østbye et al., 2005, 2006; Kahilainen et al., 2007).The whitefish Coregonus lavaretus, is the most widespread species of freshwater fish of the Northern Europe, it forms the majority of both allopatric and sympatric forms and populations, which differ in morphology, strategies of life cycle and ecological niches (Kahilainen et al., 2004, 2006, 2007, 2009, 2014; Østbye еt al., 2005; Kahilainen, Østbye, 2006; Siwertsson еt al., 2008, 2010; Harrod et al., 2010; Præbel et al., 2013 et al.). The correlation «phenotype – environment» is recognized as an important factor of adaptive radiation (Schluter, 2000). In general, it is believed that the divergence in whitefish occurs as a specialization of the resources use of the main reservoir zones: pelagial (plankton) – littoral (benthos, plankton) – profundal (benthos). These zones vary dramatically by their conditions. Littoral has got good illumination and warming, a large species diversity of food organisms; profundal is characterized by low illumination, complex gas and temperature regimes, limited benthic resources; pelagial is different from the previous structurally homogeneous habitat with available zooplankton resources. To use more effectively their specific food resources, morphological adaptations, usually associated with the configurations of the head, jaws and gill rakers, are required (Schluter, 1996). At that, the form and number of gill rakers responsible for the effectiveness of the prey retention are determining adaptive features (Решетников, 1980; Friedland et al., 2006). In whitefish the number of gill rakers is a genetically determined phenotypic trait used to separate sympatric morphs, forms, or ecotypes (Решетников, 1980). The features of the structure of intra-reservoir whitefish populations of different lakes were studied, including those experiencing different levels of anthropogenic load, allows to understand the mechanisms of population adaptation and the direction of evolutionary processes (Мина, 1986; Skúlason et al., 1999; Robinson, Parsons, 2002). In this regard, one of the largest in Northern Europe lake-river system Inari – Pasvik (Barents Sea basin) is a unique object that combines a variety of natural and anthropogenic conditions. It includes a variety of vegetation zones: taiga, forest-tundra, tundra; complicated geology of water-collecting territory (Melezhik et al., 1994), runoff regulated by a cascade of Hydroelectric power station, gradient level of industrial pollution (Moiseenko et al., 1994), the high level of aquaculture development causing invasions of new species and forms (Amundsen et al., 1999). Among the fish the most numerous species is whitefish, for which the two basic forms has been described: sparsely rakered – benthophage, living mainly in the littoral and profundal zones, and densely rakered – planktonophage that live in pelagic zone (Лукин, Кашулин, 1991; Nøst et al., 1992; Amundsen et al., 1993, 2006; Moiseenko et al., 1994; Кашулин, Решетников, 1995). Some authors also distinguish sparsely rakered and densely rakered whitefish living together with big fish of these forms (Kahilainen et al., 2004, 2006, 2007, 2009, 2014; Østbye еt al., 2005; Kahilainen, Østbye, 2006; Siwertsson еt al., 2008, 2010; Harrod et al., 2010; Præbel et al., 2013 и др.).
Oz. Kuetsjarvi is a part of the Shuonijoki river tributary system, which flows into the lower course of the Pasvik river, and is one of the most man-made polluted natural reservoirs in the Euro-Arctic region (Кашулин и др., 1999). As a result of the activity of the melting shops of the Pechenganikel metallurgical plant located on the banks of the lake in its waters (Кашулин и др., 2013; Ylikörkkö et al., 2014) and bottom sediments (Dauvalter et al., 2010; Кашулин и др., 2013) have extremely high concentrations of heavy metals. Despite the specific natural conditions and anthropogenic pollution of the lake, the transition of the whitefish to a short-cycle survival strategy allows it to maintain a high population size (Кашулин и др., 1999; Решетников и др., 1999). Here, for the first time, a population of the smallest whitefish was described, spawning in the first or second year of life when reaching the size of 7-9 cm with a life duration of three to four years (Лукин, Кашулин, 1991; Решетников и др., 1997). At the same time, there are whitefish in the lake that differ in morphology, higher size and weight indicators, late maturation periods and the absence of typical pathological transformations of internal organs and tissues. However, a detailed study of the morphology and distribution of sympatric forms of whitefish in Kuetsjarvi lake has not been held before.
The purpose of this work is to analyze the morphological features, population structure, life cycle parameters, and trophic relationships of the distinguished forms of whitefish in the Kuetsjarvi lake to assess their relationship within the reservoir.
Materials
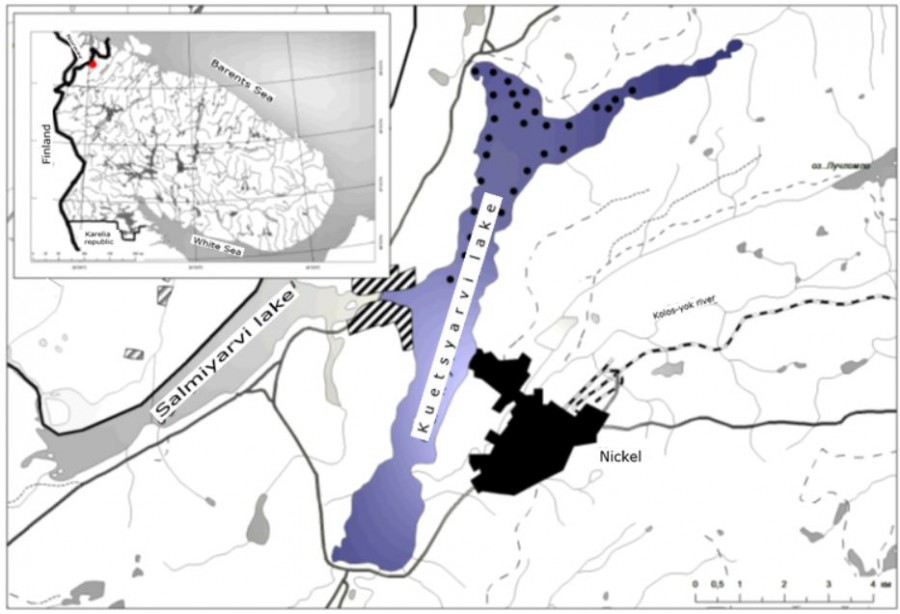
Characteristics of the reservoir. Kuetsjarvi lake (lake area 17.0 km2, maximum depth 37 m) is part of the lake-river system of the border Pasvik river, with which it is connected by a small channel (Fig. 1). The lake is of glacial origin, extended in the Meridian direction with, its length is 11.6 km, the maximum width is 2.8 km, conditional water exchange – 1.55. The shores of the lake are high, covered with pine forests and with mountain wastelands. In terms of water quality, the lake is one of the most polluted in the Murmansk region. Through the Kolos-yok river, it receives wastewater from the Pechenganikel plant. The main polluting elements are Ni, Cu, and Co, as well as the accompanying chalcophilic elements – Pb, As, Cd, and Hg. The toxic compounds (oxides of sulfur, nitrogen, heavy metals) that are released into the atmosphere and deposited on the catchment area have a great influence. The water in the lake is neutral and is characterized by values of total mineralization on average 69.0 mg/l and alkalinity on average 286 meqv/l. Ni concentrations are 106 (74-182) µg/l, Cu-8.6 (4.5–15.2) µg/l. According to the content of biogenic elements, the lake is characterized as eutrophic. The highest concentration of Робщ in the lake (up to 60 µg/l) and Nобщ (up to 390 µg/l) is observed in the summer. The values of the coefficient of contamination of bottom sediments by these elements are in the range from 33.5 to 125.7, and the degree of total pollution by heavy metals calculated for this lake is 240.1 and belongs, according to the classification of L. Hokanson (1980) (не нашел этого источника в списке литературы), to a high one. The communities of phytoplankton in Kuetsjarvi lake contains 54 species, among which diatoms are dominant, and they have high densities (more than 1700 g/m3) (Шаров, 2000). The zooplankton of the lake is highly developed, the density of Cladocera and Copepoda crustaceans is up to 80,000 exs./m3 (Яковлев и др., 1991; Noest et al., 1992). The number of zoobenthos species exceeds 20, among which chironomids dominate (60-80%). The lake's ichthyofauna includes representatives of nine species belonging to eight fish families: trout – Salmo trutta, whitefish, grouse – Coregonus albula, grayling – Thumallus thumallus, pike – Esox lucius, perch – Perca fluviatilis, burbot – Lota lota, minnow – Phoxinus phoxinus, nine-spined stickleback – Pungitius pungitius.
![]()
![]()
Fig. 1. Map-scheme of Kuetsyarvi lake and gathering sites of ichthyological samples (●) in 2015
Methods of catching fish and the amount of material collected. Fish were caught in September 2015 using standard sets of fixed gill nets from monofilament. Places of capture are shown in Fig. 1. In the littoral zone (at a depth of 1.5–3 m) nets with a length of 25 m, a height of 1.5 m, and a mesh size of 10-60 mm (which provided a catch of fish with a length of ≥ 5 cm) were installed. The nets were set in groups of 1-2 nets perpendicular to the shore in places with sand and gravel shallows and large boulder. In the profundal zone with depths of more than 18 m, up to 10 multi-cell nets in one group were used. In the pelagic zone of the reservoir, floating multi-size nets with a height of 3 m were used to select ichthyological material. Total of 199 whitefish specimens were caught.
Methods
Methods of fish processing. Each fish was numbered and photographed with a Nikon d610 digital camera with a 60 mm f/2.8G ED AF-S Micro-Nikkor lens (Бочкарева, Зуйкова, 2007). Further processing of the material was carried out according to the standard method (Сидоров, Решетников, 2014). To distinguish intraspecific groups in the studied whitefish, the rakers on the first gill arch were counted. Measurements of the length of the gill arch and the largest gill raker were performed under binoculars using an eyepiece-micrometer (Правдин, 1966). The distance between the gill rakers was calculated using the method of K. Kahilainen and K. Østbye (2006). The resulting images were used to calculate the number of punctured scales in the side line of whitefish and to measure the plastic characteristics of fish using the ImageJ program. The number and pattern of measurements were held according to K. Kahilainen and K. Østbye (2006) with minor changes. The samples were compared using the Student's t-test, as well as using the main components method in the Statistica program. Before using the methods of multidimensional statistics, the absolute values of features were transformed (Darroch, Mosimann, 1985; Mosimann, 1970), and a covariance matrix was used. The study of linear growth rates of whitefish on scales was conducted according to the method of E. M. Zubova (2015). Stomach contents were examined only in 27 specimens of whitefish caught in 2015, and 48 whitefish collected in 2012-2013, using the same method. Whitefish stomachs were extracted and fixed in a 70% solution of ethyl alcohol for 1-1.5 hours. The material was processed in the laboratory using a microscope (Методическое..., 1974). Food items were defined to the family or genus (Определитель..., 1977). To assess the size variability of feeding of whitefish from the lake, 4 size groups of whitefish were identified: 100-199 mm, 200-299 mm, 300-399 mm, and 400-499 mm (Kahilainen, Østbye, 2006).
Results
Morphology. Whitefish of the Kuetsjarvi lake is represented by two main bimodally distributed forms – sparsely rakered (hereinafter SR-sparsely raked) and densely rakered (DR-densely raked), with the number of rakers on the first gill arch, respectively, from 17 to 27 (22.7 ± 0.29) and from 27 to 42 (32.4 ± 0.23) (Fig. 2). The DR was more numerous than the SR. The ratio of the two forms in the samples averaged 2:1.
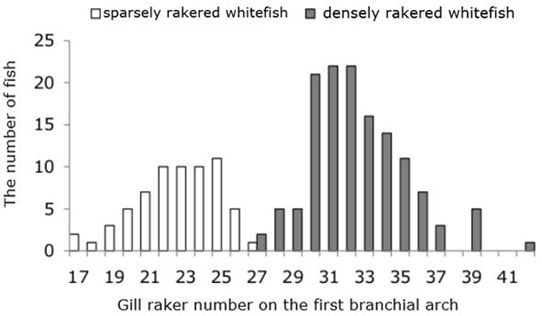
Fig. 2. The distribution of whitefish according to the gill raker number on the first branchial arch in lake Kuetsjarvi, 2015
Analysis of whitefish images allows to identify additional groups of whitefish that differ in the head structure. Among SR the following individuals was met: 1) with a large eye, a pronounced lower mouth, and a blunt snout (Fig. 3A) (onward SR1); 2) with a small eye, a semi-lower or terminal mouth, and a sharp snout (Fig. 3b) (SR2). Among DR we have met individuals: 1) with a pronounced large eye, upper mouth (Fig. 3c) (DR1); 2) with a small eye, a half-lower or terminal mouth, and a sharp snout (Fig. 3c) (DR2). At the same time, the groups of SR2 and DR2 did not differ in the morphology of the head parts (Fig. 3b, c) and their allocation is based only on the structure of the gill apparatus. The ratio of the four whitefish groups was SR1 29.1 % (58 specimens): SR2 3.5 % (7 specimens): DR1 34.7 % (69 specimens): DR2 32.7 % (65 specimens). Most of SR1 (59 %) was caught in the profundal part of the lake (table. 1), SR2 – in the littoral part (57 %); 64 % of DR1 was caught in pelagial, DR2 – in the littoral part of the lake (55 %) (see table. 1).
(a)

 (b)
(b)


(c)


(d)


Fig. 3. The appearance of sparsely rakered (a – 184 mm, 5+ years; b – 166 mm, 2+ years) and densely rakered (c – 143 mm, 5+ years; d – 179 mm, 2+ years) whitefish from lake Kuetsjarvi, 2015
Table 1. Distribution of intraspecific groups of whitefish by ecological zones of the Kuetsjarvi lake, 2015
| The place of catching | SR1 (58) | SR2 (7) | DR1 (69) | DR2 (65) |
| Littoral | 38% | 57% | 12% | 55% |
| Pelagial | 3% | - | 64% | 23% |
| Profundal | 59% | 43% | 24% | 22% |
Note. Here and in the tables and figures below: SR1 – sparsely raked whitefish 1, SR2 – sparsely raked whitefish 2, DR1 – densely raked whitefish 1, DR2 – densely raked whitefish 2; in brackets the number of individuals is shown.
In table. 2 presents 2 counting and 18 plastic morphological features of the Kuetsjarvi lake whitefish, and their mass. Sex differences on these features were not found.
Table 2. The values of some body measurements of intraspecific groups of whitefish from the Kuetsjarvi lake, 2015
| Features | SR1 (58) | SR2 (7) | DR1 (69) | DR2 (65) |
| Length (FL), mm | 169.6±4.09 | 242.4±22.38 | 114.9±1.62 | 195.3±4.40 |
| Mass, g | 54.9±4.61 | 189.6±52.57 | 14.6±0.92 | 87.2±7.32 |
| The gill raker number on the first branchial arch | 22.5±0.30 | 24.9±0.51 | 33.6±0.37 | 31.9±0.26 |
| The length of the largest gill raker, % to the length of the branchial arch | 11.1±0.23 | 12.2±0.60 | 20.1±0.41 | 15.6±0.33 |
| The distance between the rakers, mm | 0.9±0.03 | 0.8±0.11 | 0.4±0.01 | 0.7±0.02 |
| Number of punctured scales in the lateral line | 84.2±0.52 | 91.0±2.04 | 84.3±0.47 | 90.0±0.56 |
| % FL | ||||
| The length of the branchial arch | 12.0±0.16 | 11.1±0.18 | 12.7±0.14 | 11.2±0.12 |
| The length of the snout | 4.5±0.08 | 4.3±0.28 | 4.8±0.09 | 4.2±0.09 |
| The horizontal diameter of the eye | 5.4±0.08 | 4.3±0.22 | 6.1±0.06 | 4.6±0.06 |
| The vertical diameter of the eye | 5.3±0.16 | 4.5±0.57 | 6.0±0.09 | 4.7±0.14 |
| The postorbital part of the head | 10.7±0.14 | 9.7±0.32 | 10.3±0.15 | 9.6±0.13 |
| The length of the upper jaw | 5.9±0.09 | 5.1±0.28 | 7.2±0.12 | 5.3±0.09 |
| The length of the lower jaw | 8.6±0.13 | 7.9±0.37 | 10.1±0.14 | 8.1±0.13 |
| The length of the head | 19.9±0.24 | 17.8±0.82 | 21.1±0.30 | 17.7±0.24 |
| The head height at a nape | 14.8±0.12 | 13.3±0.23 | 14.4±0.09 | 13.4±0.09 |
| The greatest body height | 21.3±0.22 | 22.0±0.85 | 19.0±0.17 | 20.5±0.22 |
| The smallest body height | 6.7±0.04 | 7.3±0.18 | 6.4±0.03 | 6.8±0.04 |
| The length of the dorsal fin | 16.7±0.25 | 16.5±0.56 | 16.0±0.28 | 15.6±0.21 |
| The length of the pectoral fin | 16.6±0.30 | 14.6±0.62 | 15.9±0.27 | 13.9±0.20 |
| The length of the ventral fin | 14.0±0.26 | 13.0±0.64 | 13.4±0.25 | 12.8±0.20 |
| The length of the anal fin | 9.6±0.18 | 8.9±0.38 | 8.8±0.17 | 8.6±0.16 |
Note. Here and in tables 3, 4, 6, 7 the average values of the features and their errors are shows.
The selected whitefish groups differed (p < 0.01) in linear-weight characteristics, and they decreased in a row: SR2 > SR2 > SR1 > DR1 (see table. 2).
The structure of the gill apparatus (the number of rakers on the first branchial arch, the relative lengths of the largest gill raker and the gill arch) of the four whitefish groups differed (p < 0.05): DR1 > DR2 > SR2 > SR1 (see table. 2). The distance between the rakers was significantly greater in SR1 and SR2. A diagram of frequency distribution of the number of rakers in the four whitefish groups is shown in Fig. 4.

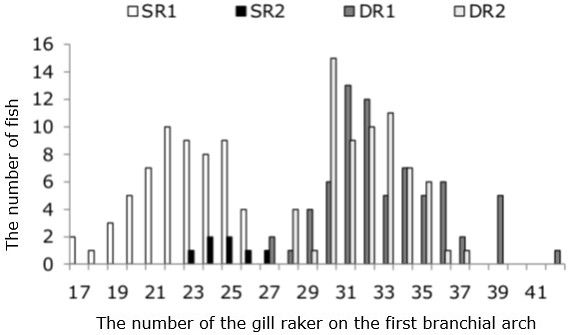
Fig. 4. The distribution of intraspecific groups of whitefish according to the gill raker number on the first branchial arch from lake Kuetsjarvi, 2015
The relative indicators of head morphology in SR2 and DR2 did not differ significantly, while the SR1 and DR1 in whitefish groupings differed in head structure from each other and from SR2-DR2 (see table. 2). Thus, the DR1 group had the largest horizontal and vertical diameter of the eye (p < 0.001), slightly smaller diameter had SR1, SR2 and DR2 (see table. 2). The same can be said about other head indicators, except postorbital part of the head and its height, which were significantly more in SR1 than in DR1. The highest relative body height indicators were typical for SR whitefish, the lowest for DR1 (p < 0.05). All whitefish groups had different relative pectoral fin lengths (p < 0.05): SR1 > DR1 > DR2 > DR2. By the number of punctured scales in the lateral line only two groups of whitefish were distinguished (p < 0.001): on average, 84 scales are typical for SR1 and DR1, and a large number of scales: 90-91-for SR2 and DR2 (see table. 2).
The obtained analysis of some indices of plastic and meristic morphological characters of whitefish confirms the conclusions that, basing of size and weight indicators, the structure of the first branchial arch and the length of the pectoral fin, four intraspecific groups of whitefish can be distinguished: SR1, SR2, DR1 and DR2; basing on the head morphology – three: SR1, SR1 and SR2-DR2. The number of scales in the sideline defines two groups: DR1-DR1 and DR2-DR2.
According to the results of the analysis of the contribution of plastic features into the main components (ГК), it is worth considering only the ГК2 graph against ГК14; ГК3 and ГК4 have low factorial loads (Table 3). On the graph (Fig. 5), three whitefish groups are clearly distinguished: SR1, DR1 and DR2.
Based on the results of the analysis of the contribution of plastic features to the main components (ГК), it is worth considering only the graph ГК2 against ГК1, ГК3 and ГК4 have low factorial loads (table. 3). On the graph (Fig. 5) three whitefish groupings are clearly distinguished: SR1, DR1, and DR2.
Table 3. Contributions of plastic features to the main components 1-4 in intraspecific groups of whitefish from Kuetsjarvi lake, 2015
| Features | ГК | |||
| 1 | 2 | 3 | 4 | |
| The length of the snout | 0.211 | -0.411 | -0.499 | -0.659 |
| The horizontal diameter of the eye | 0.735 | 0.177 | -0.138 | 0.505 |
| The vertical diameter of the eye | 0.835 | 0.384 | 0.361 | -0.149 |
| The postorbital part of the head | -0.341 | -0.238 | 0.062 | -0.312 |
| The length of the upper jaw | 0.705 | -0.364 | -0.411 | 0.170 |
| The length of the lower jaw | 0.582 | -0.258 | -0.316 | 0.071 |
| The length of the head | 0.490 | -0.375 | -0.239 | -0.112 |
| The head height at a nape | -0.426 | 0.695 | -0.196 | 0.265 |
| The length of the dorsal fin | -0.619 | -0.403 | 0.407 | 0.010 |
| The length of the pectoral fin | -0.358 | -0.607 | 0.288 | 0.262 |
| The length of the ventral fin | -0.565 | -0.516 | 0.372 | 0.078 |
| The length of the anal fin | -0.635 | -0.482 | 0.413 | -0.014 |
| The greatest body height | -0.745 | 0.599 | -0.158 | -0.030 |
| The smallest body height | -0.592 | 0.722 | -0.115 | -0.066 |
| Eigenvalue, % | 43.96 | 22.74 | 10.34 | 6.49 |
Note. In bold, the maximum contributions of features are highlighted. The length of the eigenvector is equal to 1.
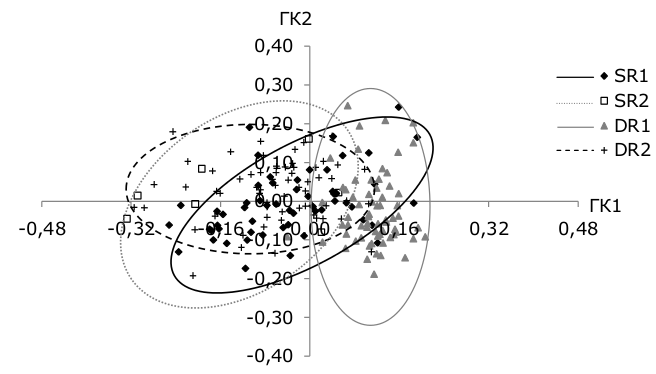

Fig. 5. The location of whitefish individuals in the space of 1–2 main components according to plastic features from Kuetsjarvi lake, 2015
The main positive contribution to the first major component was made by head shape features (the size of the eye and upper jaw), and the main negative contribution was made by body shape features (the length of the anal fin, the highest and lowest body height) (see table. 3). The main positive contribution to the second main component was made by the features of the body complex (the highest and lowest body height) and the head (the height of the head at the back of the head), the main negative – the length of the pectoral fin (see table. 3). SR2 whitefish do not form a separate group on the main component graph (see Fig. 5) and they belong more to the DR2 group, which is confirmed by the analysis of indices of morphological features of whitefish of the Kuetsjarvi lake.
Growth rate and size-age structure. The SR1 and SR2 groups were represented by individuals aged respectively 1+ to 9+ and 2+...6+ years (table. 4). In the first group the fish aged 4+...6+ (60 %) dominate, in the second – the domination is unknown due to the small number of fish. The actual length and mass of SR2 whitefish was significantly higher in all ages compared to SR1. DR1 and DR2. They were represented by individuals aged 2+ to 6+ and 1+, respectively...9+ (see table. 4). Both in the first and the second groups the fish aged 2+ to 4+ (75-96 %) dominate. As in SR whitefish, the size and weight characters of DR2 whitefish were higher in all ages compared to DR1. This led to a more detailed study of the size characters of the selected groups of whitefish in Kuetsjarvi lake.
To identify the features of the growth rate of whitefish, a single point diagram of the body length–the length of the scales radius was constructed, which is equally well described by both linear and power function equations (Fig. 6a, b). For the inverse calculations, the coefficients of the equation of the linear function were used, the lengths were calculated by the Rosa Lee formula (Брюзгин, 1969; Мина, 1973; Зубова, 2015).

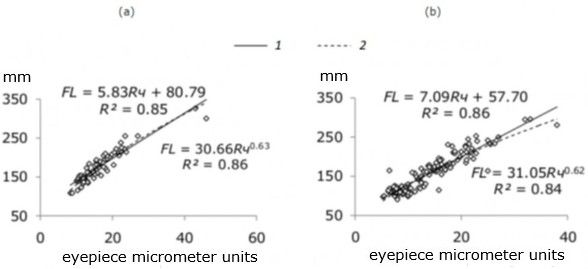
Fig. 6. Comparison of the actual body length (FL) and the front diagonal radius of scales (Rч) of sparsely rakered (a) and densely rakered (b) whitefish from Kuetsjarvi lake, 2015
In the results of inverse calculations of the length using the regression method, the Li «phenomenon» does not appear. Sex differences in growth rate was not found. In the study of «individual» linear growth rates in SR-and DR-whitefish starting from the second or third year of life, slow-growing (SR1 and DR1) and fast-growing (SR2 and DR2) groups are clearly determined. The average values of the calculated whitefish length are shown in table. 4. Both the actual and calculated length of whitefish groups in different ages significantly differed (p < 0.01), the distribution of fish in the row according to the actual and calculated length was similar: SR2 > DR2 > SR1 > DR1. Starting from the second year of life, estimates of calculated length based on relative increments are similar to estimates based on absolute increments (table. 4).
Table 4. Actual length (FL), mm, mass, g, values of calculated length, mm, absolute annual increments, mm, and specific growth rate (S) of intraspecific whitefish groups from Kuetsjarvi lake
| Intraspecific whitefish groups | Age, years | ||||||||
| 1+ | 2+ | 3+ | 4+ | 5+ | 6+ | 7+ | 8+ | 9+ | |
| Length
Mass |
|||||||||
| SR1 | 123±14.0 18±6.5 (2) | 137±7.4 25±4.6 (6) | 140±4.8 25±2.4 (7) | 159±6.4 43±6.5 (10) | 173±4.4 51±4.2 (15) | 193±7.5 80±9.4 (10) | 207±6.6 107±19.4 (4) | 215 115 (1) | 219±6.0 119±18.0 (2) |
| SR2 | - | 166 47 (1) | 198±16.5 83±20.1 (2) | 255 194 (1) | 291±35.5 287±118.5 (2) | 300 348 (1) | - | - | |
| DR1 | - | 114±2.4 14±1.3 (32) | 114±3.2 14±1.8 (19) | 116±1.4 15±0.5 (15) | 137±6.0 25±1.0 (2) | 164 45 (1) | - | - | |
| DR2 | 165 41 (1) | 171±2.3 49±2.1 (26) | 179±4.9 60±5.3 (19) | 219±4.5 110±8.4 (11) | 232±6.5 138±11.0 (8) | 246±14.3 173±36.7 (6) | - | - | 295 290 (1) |
| Calculated length | |||||||||
| SR1 | 105±0.7 (57) | 117±0.9 (55) | 129±1.3 (49) | 143±1.9 (42) | 157±2.2 (32) | 170±5.3 (17) | 182±4.0 (7) | 191±5.3 (3) | 196±0.3 (2) |
| SR2 | 115±5.5 (7) | 138±4.2 (7) | 169±8.2 (6) | 212±11.8 (4) | 239±19.0 (3) | 257 (1) | - | - | - |
| DR1 | 87±0.8 (69) | 98±1.1 (69) | 104±1.4 (37) | 113±2.2 (18) | 133±10.1 (3) | 160 (1) | - | - | - |
| DR2 | 95±1.4 (65) | 123±1.7 (64) | 142±2.0 (38) | 170±3.1 (26) | 195±4.8 (15) | 207±7.2 (17) | 228 (1) | 273 (1) | 288 (1) |
| Absolute annual growth increase / specific growth rate | |||||||||
| SR1 | 105 / - | 12/0.11 | 12/0.10 | 14/0.10 | 14/0.09 | 13/0.08 | 12/0.07 | 9/0.05 | 5/0.03 |
| SR2 | 115 / - | 23/0.18 | 31/0.20 | 43/0.23 | 27/0.12 | 18/0.07 | - /- | - / - | - / - |
| DR1 | 87 / - | 11/0.12 | 6/0.06 | 9/0.08 | 20/0.16 | 27/0.18 | - / - | - / - | - / - |
| DR2 | 95 / - | 28/0.26 | 19/0.14 | 28/0.18 | 25/0.14 | 12/0.06 | 21/0.10 | 45/0.18 | 15/0.05 |
Nutrition. The analysis of whitefish nutrition showed that in SR1 < 200 mm long, the main part of the food lump (50 %) consists of the representatives of profundal benthos: chironomid larvae (Chigopomis sp., Micropsetra sp., Sergentia sp., Procladius sp., Prodiamesa sp., Strictochironomus sp.) and bivalves of the genus Euglesa sp. (table. 5). Zooplankton organisms were found in the stomachs of 46 % of individuals of this size group and were represented by the bottom copepod crustaceans Acanthocyclops sp. (21 % of the food lump). Individual specimens had water mites. With increasing of linear characters, SR1 whitefish fed on the same groups of invertebrates, but the value of zooplankton organisms in the diet decreased, and chironomid larvae were already 77 % of the food lump (see table. 5). The size of food components increases with the growth of SR1, and the relative size of the food lump decreases.
Table 5. Feeding of intraspecific groups of whitefish in the summer-autumn period from the Kuetsjarvi lake, 2012-2015
| - | SR1 SR2 DR1 DR2 | ||||||||
| Size groups (FL), mm | |||||||||
| 100–199 | 200–299 | 200–299 | 300–399 | 400–499 | 100–199 | 100–199 | 200–299 | 300–399 | |
| - | P, % / F, % | P, % / F, % | P, % / F, % | P, % / F, % | P, % / F, % | P, % / F, % | P, % / F, % | P, % / F, % | P, % / F, % |
| Zooplankton | 20.9 / 46 | 3.0 / 33 | - | - | - | - | 37.8 / 40 | 16.6 / 7 | - |
| Benthos | 50.3 / 65 | 82.3 / 100 | 100 / 100 | 94.0 / 100 | 100 / 100 | ||||
| Caddis flies (pupae, larvae) | - | - | 63.4 / 100 | 83.7 / 100 | 99.0 / 100 | - | 22.2 / 60 | 27.2 / 27 | 100 / 100 |
| Chironomids (pupae, larvae) | 43.9 / 65 | 76.7 / 100 | - | - | 0.8 / 100 | 26.3 / 100 | 39.7 / 60 | 35.2 / 60 | - |
| Hymenoptera (imago) | - | - | - | - | - | - | - | 0.03 / 7 | - |
| Hemiptera (imago) | - | - | - | - | - | - | - | 0.03 / 7 | - |
| Lepidoptera (imago) | - | - | - | - | - | - | - | 0.1 / 7 | - |
| Beetles (imago) | - | - | - | 0.2 / 7 | - | ||||
| The water mites (adults) | 0.1 / 4 | - | - | - | 0.2 / 100 | - | - | 0.04 / 27 | - |
| Mollusca | 6.3 / 23 | 5.6 / 22 | 36.6 / 50 | 10.3 / 33 | - | - | 0.3 / 10 | 5.7 / 7 | - |
| The remains of insects | - | - | - | - | - | - | - | 3.3 / 7 | - |
| Fish | - | - | - | - | - | - | - | 7.3 / 7 | - |
| Fish roe | - | - | - | 6.0 / 33 | - | - | - | - | |
| An amorphous mass | 28.8 / 39 | 14.7 / 22 | - | - | - | 73.7 / 100 | - | 4.3 / 13 | - |
| Average size of forage organisms, mm | 7.3 1.2– 16.0 | 8.6 1.0– 16.4 | 9.7 3.8 – 20.0 | 6.3 2.7– 12.0 | 6.3 1.0– 9.5 | - | 7.9 0.8– 22.0 | 9.5 0.6– 34.0 | 7.1 6.0– 8.1 |
| Average weight of a food lump, mg | 235 | 404 | 1011 | 7749 | 1031 | 95 | 235 | 842 | 6169 |
| Average In, 0/000 | 45.9 | 37.1 | 35.2 | 205.9 | 12.2 | 40.0 | 49.8 | 52.6 | 177.3 |
| Number of individuals | 26 | 9 | 2 | 3 | 1 | 1 | 10 | 15 | 1 |
In fast-growing SR2, the stomachs of individuals of 3 size groups were examined: from 200 to 499 mm (see table. 5). In the stomachs of these whitefish mainly representatives of littoral benthos were found – the caddis flies larvae of Agraylea sp., Athripsodes sp., Mystacides sp. and Molana sp. (> 1 thousand individuals in one stomach, from 63 to 99 % of the mass of the food lump) and gastropods Limnea sp. and Valvata sp. (from 10 to 37 % of the food lump) (see table. 5). There were also small numbers of chironomid larvae and bivalves, fish roe. The fast-growing SR2 also has a tendency to increase the size of food components and food lumps with growth.
The fast-growing DR2 had a higher nutritional diversity than the SR-whitefish (see table. 5). In DR2 < 200 mm long, the main part of the food lump was made up of representatives of profundal benthos (chironomid larvae belonging to 8 genera) and zooplankton organisms (large bottom crustaceans of the genera Acanthocyclops sp. and Eurycercus sp.) (see table. 5). In the stomachs of 60% of DR2-whitefish of this size group, representatives of littoral benthos were also found – larvae of сaddis flies of the Phryganeidae and Leptoceridae families (22 % of the weight of the food lump) (see table. 5). With an increase in size (from 200 to 299 mm), this group of whitefish mainly fed on chironomid larvae (10 genera) and сaddis flies (3 families) (see table. 5). Zooplankton organisms were found only in 1 specimen of whitefish (Eurycernus sp., Bosmina sp.), they can also be attributed to gastropods of the genus Limnea sp. In the stomachs of some individuals, imagos of 5 orders of insects were found (see table. 5). Fish (nine-spined stickleback) was found in the food of this DR2 size group. In a stomach of DR2 specimen longer than 299 mm, only сaddis flies’ larvae were found (Agraylea sp., Mystacides sp.). The absolute and relative masses of the food lump of fast-growing DR2 are close to those of fast-growing SR2 whitefish and increase with the growth of fish (see table. 5).
In the stomach of a slow-growing DR1 whitefish < 200 mm long, chironomid larvae (Chigopomis sp.) were determined (see table. 5).
Despite the high number of rotifers in the zooplankton of Kuetsjarvi lake and oligochaet as part of profundal benthos (Environmental..., 2014), they were rarely or not found in the stomachs of the fish studied.
Maturation. The slow-growing DR1 whitefish begin to mature at the smallest size and weight compared to other intraspecific groups of whitefish – at a length of 100-104 mm, mass 7-8 g, aged 2+, in bulk with an average length of 117 mm and a mass of 15 g at the same age. In fast-growing DR2 individuals matured at large sizes: with a length of 174-191 mm and a weight of 56-82 g at the age of 2+...3+, mass at an average length of 220 mm and a mass of 117 g at the age of 4+mm...5+. Individuals of slow-growing SR1s began to mature at a length and weight of 116-135 mm and 13-22 g, respectively, at the age of 2+...3+, mass – with an average length and weight of 172 mm and 51 g at the age of 4+...5+. Among fast-growing SR2s there were no sexually matured.
Discussion
The phenomenon of a high number of oligotoxobic species Coregonus lavaretus in a heavily polluted Kuetsjarvi lake (69-96 % of catches over the past 20 years) was described in details earlier (Кашулин и др., 1999), and in this paper we do not consider the impact of pollution on structural indicators of populations. At the same time, the study of morphophysiological differences in sympathetic forms expands our understanding of the adaptation capabilities of whitefish to adverse environmental conditions. Our research allows us to distinguish in Kuetsjarvi lake four groups of whitefish that differ in the combination of plastic and meristic features, size and weight indicators, and, accordingly, growth rates: SR1-slow-growing sparsely raked whitefish with large eyes, pronounced lower mouth, blunt snout, and fast-growing sparsely raked whitefish with small eyes, a half-lower or terminal mouth, sharp snout; DR1 – slow growing densely raked whitefish with distinct large eyes, the upper mouth; DR2 – fast-growing densely raked whitefish with small eyes, a half-lower or terminal mouth. All distinguished groups of whitefish had significant differences in the structure of the gill apparatus. Slow-growing S R1 and DR1 whitefish form a medium-scale group of whitefish in the Kuetsjarvi lake, fast-growing groups of SR2 and DR2 whitefish – multi-scale group (Бочкарев, Зуйкова, 2010). SR1 mainly lives in the profundal zone of the lake, DR1 – in the pelagic zone; similar in external structure SR2 and DR2 – in the littoral part of the lake. Thus, individual groups of features often overlap in different groups of whitefish of the Kuetsjarvi lake.
It is believed that such a feature as the number of punctured scales in the lateral line cannot be considered as adaptive to environmental conditions, and the feature itself can be used as a marker when studying the settlement of various whitefish lines (Бочкарев, Зуйкова, 2010). At this stage, we cannot speculate about the phylogenetic lines of the whitefish in Kuetsjarvi lake, because such research in the Murmansk region has not been carried out.
If in the classification of whitefish of Kuetsjarvi lake one take into account such a stable sign as the gill raker number on the first branchial arch, and the values of plastic signs, then we can say that Kuetsjarvi lake is inhabited by SR- and DR-whitefish, and the differences in external structure described above between SR1 and SR2, as well as between DR1 and DR2, are the result of different individual ontogenetic allometry within these groups, caused by differences in their growth rate. Measurements of head parts in fast-growing whitefish groups SR2 and DR2 of the Kuetsjarvi lake have significantly smaller values than in individuals of slow-growing SR1 and DR1 whitefish in the same age groups (Fig. 7a, b). The dependence of the exterior of whitefish and other fish species on their growth rates was noted by some authors earlier (Мина, 1986; Решетников, 1980; Алексеев и др., 2014).
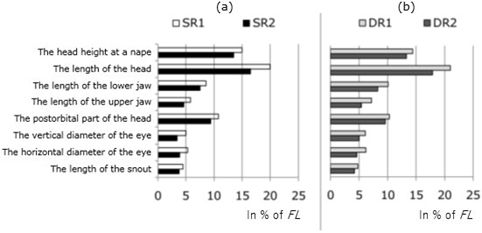
Fig. 7. Comparison of head size (% FL) in slowly growing (SR1) and fast-growing (SR2) sparsely rakered whitefish at the age of 4+...6+ (a) and in slowly growing (DR1) and fast-growing (DR2) densely rakered whitefish at the age of 2+...4+ (b) from lake Kuetsjarvi, 2015
A number of researchers have shown that significant differences in the growth rate of distinguished groups of whitefish in the Pasvik river system are determined by the quality and quantity of food items in the diet of fish confined to a particular area of the reservoir (Kahilainen et al., 2003, 2004; Kahilainen, Østbye, 2006). According to our results, the differences in the growth rates of the selected SR and DR whitefish groups in the Kuetsjarvi lake, especially from the 2-3 years of life, are the result primarily of different quality of fish feeding and are not related to the number of food items. Thus, the relative size of the food lump in large whitefish SR and DR (SR2, DR2) was larger than in small whitefish (SR1, DR1), only in individuals with sizes > 299 mm (older than 5+ years) (see table. 5). In SR1, which lives in the profundal zone of Kuetsjarvi lake, representatives of the profundal macrozoobenthos were found in the stomachs: larvae of chironomids, which form the basis of the deep macrozoobenthos of Kuetsjarvi lake, bivalve mollusks and large bottom-level zooplankton. While SR2, which lives in the littoral zone, had representatives of the littoral benthos in their stomachs: caddis fly larvae and gastropods. Representatives of littoral benthos are probably the most energy-efficient type of food than profundal macrozoobenthos. Despite the fact that 55 % of DR2 was caught in the littoral zone of the lake, the contents of their stomachs included representatives of not only littoral (caddis fly larvae), but profundal macrozoobenthos, bottom and pelagic zooplankton, and fish. This can be explained by the fact that the remaining 23 and 22 % of the fish of this form were caught in the pelagic and profundal zones of Kuetsjarvi lake, i.e. DR2 whitefish is a pronounced euryphage compared to SR whitefish. As for DR1, we can assume that this group of whitefish mainly feeds on pelagic zooplankton, since 64 % of individuals live in this area of the lake. The pelagic zooplankton of Kuetsjarvi lake is more than 97 % represented by rotifers (Rotatoria), which are probably low-value food items, as well as chironomids, compared to crustaceans (Cladocera and Sopepoda), which determines the slowest growth rate in these groups of whitefish. DR1 can also feed on profundal macrozoobenthos – 25 % of whitefish of this group were caught in the profundal zone of the lake. Chironomid larvae were found in the stomach of the only individual of this group of whitefish examined for food. The reason for the slow growth of DR1 in the Kuetsjarv lakei, as in Mudusjärvi lake (Зубова, 2015; Kahilainen, Østbye, 2006), may be its high population number compared to SR whitefish, as well as its nutritional competition with the introduced Kuetsjärvi grouse, which is a pronounced pelagic fish and feeds on zooplankton.
The peculiarity in the exterior of the four intraspecific groups of the whitefish of the Kuetsjarvi lake also play a functional role in the feeding and movement of fish confined to certain lake areas. Thus, the largest eye diameter and the «large» upper mouth of the DR1 whitefish are typical features of planktophages that live in the water column (Никольский, 1974), and the lowest body height is energetically advantageous when searching for and extracting zooplankton organisms in pelagial (Kahilainen, Østbye, 2006). At the same time, the relatively large diameter of the eye in SR1 may contribute to finding food in low light at the depths of the lake, and the lower location of the mouth is effective when feeding on profundal macrozoobenthos (Никольский, 1974). Half-lower and terminal mouth of big whitefish of Kuetsjarvi lake SR2-DR2 is probably the most versatile, and the presented groups of whitefish can eat different types of food or be larger adapted for feeding on littoral benthos, as we have shown above and noted for large whitefish in other lakes of the Pasvik river system (Kahilainen, Østbye, 2006).
The structure of the first gill arch should play a major role in feeding of fish (Решетников, 1980; Kahilainen, Østbye, 2006). The intraspecific groups of whitefish SR (SR1 and SR2) and whitefish DR (DR1 and DR2) in Kuetsjarvi lake in average have very close values of gill raker number on the first branchial arch (see table 2) and plays a secondary role in their separation (see above). According to our data, SR whitefish in the Kuetsjarvi lake are mainly benthic, while DR2 have a mixed diet, and in DR1 food zooplankton organisms may dominate. In general, the average size of organisms in the stomachs of intraspecific groups of whitefish and Kuetsjarvi lake had similar values (see table. 5), the minimum size of food organisms exceeded the minimum distance between the rakers (see table. 2, 5). Approximately the same average size of invertebrates was found in the stomachs of DR2 (see table. 2), although the size range was higher, unlike SR whitefish. The minimum size of food organisms in DR2 also exceeded the minimum distance between the rakers (see table. 2, 5).
Our research has shown (Зубова, 2015) that the onset of sexual maturity in whitefish both in different reservoirs and within the same reservoir in the Murmansk region can occur at very different lengths and at different ages. The relationship between maturation and growth rates of intraspecific groups of whitefish in Kuetsjarvi lake is a form of relationship between the growth rate and the rate of puberty of fish (Лапин, Юровицкий, 1959). The reason for this may be the different quality of food in four intraspecific groups of whitefish in Kuetsjarvi lake (see above) (Лапин, Юровицкий, 1959; Кошелев, 1971). It is possible that the low caloric content of food items in slow-growing whitefish leads to the onset of sexual maturity at smaller sizes than in fast-growing forms, which was also observed in other fish species (Кошелев, 1971). The size of intraspecific groups of whitefish in which mass maturation occurred is close to the size of mass spawning of whitefish of the same groups from other reservoirs of the Pasvik river system (Kahilainen et al., 2004; Зубова, 2015).
The results we obtained for whitefish from the Kuetsjarvi lake are close to the results found for the upper and middle stream whitefish of the Pasvik river system: slow-growing (SR1) and fast-growing (SR2) sparsely rakered whitefish of Kuetsjarvi lake correspond to those of SSR (small sparsely rakered) and LSR (large sparsely rakered); slow-growing densely rakered whitefish (DR1) corresponds to DR (densely rakered), fast-growing – LDR (large densely raked) (Østbye et al., 2005; Kahilainen, Østbye, 2006; Kahilainen et al., 2004, 2006, 2007, 2009, 2014; Harrod et al., 2010; Siwertsson et al., 2008, 2010; Præbel et al., 2013). At the same time, the average number of rakeres in the SSR (SR1) on the first branch arch increases from the upstream of the Pasvik to the dowbstream: from 17 (Mudusjarvi lake) to 22 rakeres (Kuetsjarvi lake). In other groups of whitefish from different parts of the system, the number of rakeres on the first gill arch varies slightly: in SR2 (LSR) – on average from 23 to 25 rakeres, in DR1(DR) – from 34 to 36 rakeres, in DR2 (LDR) – from 32 to 34 rakeres. This leads to the fact that the distinguished four groups of whitefish of the Kuetsjarvi lake are the closest in the number of rakeres on the first Gill arch than in the rest of Pasvik.
Conclusions
In Kuetsjarvi lake (Murmansk district), which is one of the most industrially polluted natural waters Euro-Arctic region, four intraspecific groups of whitefish have been distinguished: slow-growing sparsely rakered whitefish, fast-growing sparsely rakered whitefish, slow growing densely rakered whitefish, fast growing densely rakered whitefish. The specialization of these whitefish on a certain type of resources causes their morphological and behavioral differences, spatial differentiation and, ultimately, different ecological niches.
References
Østbye K, Amundsen P-A, Bernatchez L. et al. Parallel evolution of ecomorphological traits in the European whitefish Coregonus lavaretus (L.) species complex during postglacial times, Mol. Ecol. 2006. Vol. 15. P. 3983–4001.
Østbye K., Naesje T, F., Bernatchez L. et al. Morphological divergence and origin of sympatric populations of European whitefish (Coregonus lavaretus (L.) in Lake Femud, Norway, J. Evol. Biol. 2005. Vol. 18. P. 683–702.
Alekseev S. S. Gordeeva N. V. Matveev A. N. Three sympatric forms of arctic charr Salvelinus alpinus Complex (Salmoniformes, Salmonidae) from Lake Kamkanda, Northern Transbaikalia, Voprosy ihtiologii. 2014. T. 54. No. 4. P. 387–412.
Amundsen P. A., Kashulin N. A., Koroleva I. M. et al. Environmental monitoring of fish in the Paz watercourse. NCFS, University of Tromse, Norway, 2006. 48 p.
Amundsen, P. A., Staldvik, F., Lukin, A. et al. Ecology and heavy metal contaminations in the fish communities of the Pasvik River System. Report. Norwegian College of Fishery Science. University of Tromsø, Norway, 1993. 29 p.
Bernatchez L. Ecological theory of adaptive radiation: empirical assessment from Coregonine fishes (Salmoniformes), Hendry AP, Stearns SC (eds.). Evolution illuminated: salmon and their relatives. Oxford: Oxford University press, 2004. P. 175–207.
Bochkarev N. A. Zuykova E. I. Politov D. V. New options for collection and documentation of morphological data in fish, Biologiya, biotehnika razvedeniya i sostoyanie zapasov sigovyh ryb: Materialy mezhdunar. konf. Tyumen': FGUP «Gosrybcentr», 2013. P. 32–36.
Bochkarev N. A. Zuykova E. I. Comparative characteristic of whitefish (Coregonus lavaretus pidschian, Coregonidae) from the lake Karakul and the river Bolshoy Abakan - to the question of secondary intergradation of whitefish in the upper and middle basin of the river Yenisei, Trudy ISiEZh SO RAN. 2010. Vyp. 46. P. 198–224.
Bryuzgin V. L. Methods of studying fish growth by scales, bones and otoliths. Kiev: Naukova dumka, 1969. 188 p.
Dauvalter V. A., Dauvalter M. V., Kashulin N. A. et al. Chemical composition of bottom sedimentary deposits in lakes in the zone impacted by atmospheric emissions from the Severonickel plant, Geochemistry International. 2010. Vol. 48. No 11. P. 1224–1229.
Doroch J. N., Mosimann J. E. Canonical and principal components of shape, Biometrika. 1985. Vol. 72. No 2. P. 241–252.
Friedland K. D., Ahrenholz D. W., Smith J. W. et al. Sieving functional morphology of the gill raker feeding apparatus of Atlantic menhaden, J. Exp. Zool. 2006. Vol. 305A. P. 974–985.
Harrod C., Mallela L., Kahilainen K. Phenotype-environment correlations in a putative whitefish adaptive radiation, J. of Animal Ecol. 2010. Vol. 79. P. 1057–1068.
Kahilainen K., Østbye K. Morphological differentiation and resource polymorphism in three sympatric whitefish Coregonus lavaretus (L.) forms in a subarctic lake, J. Fish Biol. 2006. Vol. 68. P. 63–79.
Kahilainen K., Alajärvi E., Lehtonen H. Planktivory and diet-overlap of densely rakered whitefish (Coregonus lavaretus(L.)) in a subarctic lake, Ecol. Freshw. Fish. 2005. Vol. 14. P. 50–58.
Kahilainen K., Lehtonen H., Könönen K. Consequence of habitat segregation to growth rate of two sparsely rakered whitefish (Coregonus lavaretus (L.)) forms in a subarctic lake, Ecol. Freshw. Fish. 2003. Vol. 12. P. 275–285.
Kahilainen K., Malinen T., Lentonen H. Polar light regime and piscivory govern diel vertical migrations of planktivorous fish and zooplankton in a subarctic lake, Ecol. Freshwater Fish. 2009. Vol. 18. P. 481–490.
Kahilainen K., Malinen T., Tuamaala A. et al. Diel and seasonal habitat and food segregation of three sympatric Coregonus lavaretus forms in a subarctic lake, J. Fish Biol. 2004. Vol. 64. P. 418–434.
Kahilainen K., Malinen T., Tuomaala A. et al. Empirical evaluation of phenotype–environment correlation and trait utility with allopatric and sympatric whitefish, Coregonus lavaretus (L.), populations in subarctic lakes, Biol. J. Linn. Soc. 2007. Vol. 92. No 3. P. 561–572.
Kahilainen K., Patterson W., Sonninen E. et al. Adaptive Radiation along a Thermal gradient: preliminary results of habitat use and respiration rate divergence among whitefish morphs, Plos One. 2014. Vol. 11. No 9. P. 1–12.
Kashulin N. A. Dauval'dar V. A. Denisov D. B. Some aspects of the current state of freshwater resources of Murmansk region, Vestnik MGTU. 2013. T. 16. No. 1. P. 98–107.
Kashulin N. A. Lukin A. A. Amundsen P. Fish of Subarctic freshwater system as bioindications of industrial pollution. Apatity: Izd-vo KNC RAN, 1999. 142 p.
Kashulin N. A. Reshetnikov Yu. S. Accumulation and distribution of nickel, copper and zinc in the organs and tissues of fish in subarctic reservoirs, Voprosy ihtiologii. 1995. T. 35. No. 5. P. 687–697.
Key to freshwater invertebrates in the European part of the USSR (plankton and benthos), Pod red. L. A. Kutikova, Ya. I. Starobogatova. L.: Gidrometeoizdat, 1977. 511 p.
Klemetsen A., Elliott J. M., Knudsen R. et al. Evidence for genetic differences in the offspring of two sympatric morphs of Arctic charr, J. Fish. Biol. 2002. Vol. 60. P. 933–950.
Koshelev B. V. Some regularities of growth and time of the onset of the first spawning in fish, Zakonomernosti rosta i sozrevaniya ryb. M.: Nauka, 1971. P. 186–218.
Lapin Yu. E. Yurovickiy Yu. G. About intraspecific patterns of maturation and fertility dynamics in fish, Zhurnal obschey biologii. 1959. T. 20. No. 6. P. 439–446.
Lukin A. A. Kashulin N. A. The ichthyofauna status of water bodies in the border zone of the USSR and Norway. Apatity: Izd-vo KNC RAN, 1991. 50 p.
Melezhik V. A. et al. Pechenga area, Russia. Part 1: geological setting and comparison with Pasvik, Norway, Trans. Instit. Min. Metall: Sect. B: Appl. Earth. Sci. 1994. Vol. 103. P. 129–165.
Methodical manual on the study of nutrition and nutritional relationships of fish in natural conditions. M.: Nauka, 1974. 254 p.
Mina M. V. Fish growth (research methods in natural populations), Rost zhivotnyh. Zoologiya pozvonochnyh. M.: VINITI, 1973. T. 4. P. 68–115.
Mina M. V. Fish microevolution: evolutionary aspects of phenetic diversity. M.: Nauka, 1986. 193 p.
Moiseenko T., Mjelde M., Brandrud T. et al. Pasvik River Watercourse, Barents region: pollution impacts and ecological responses, Investigation in 1993 Institute of North Indusrial Ecology Problems (Russia), Norwegian Institute for Water Research (Norwey). NIVA-report OR-318. Oslo, 1994. P. 1–87.
Mosimann J. E. Size allometry: Size and shape variables with characterizations of the lognormal and generalized gamma distributions, J. Am. Stat. Ass. 1970. Vol. 65. P. 930–945.
Nøst T., Yakovlev V., Berger H. M. et al. Impacts of pollution on freshwater communities in the border region between Russia and Norway. NINA Scientific Report 26. Norwegian Institute for Nature Research. Trondheim, Norway, 1991. 41 p.
Nikol'skiy G. V. Theory of fish herd dynamics. M.: Pisch. prom-st', 1974. 447 p.
Præbel K., Knudsen R., Siwertsson A. et al. Ecological speciation in postglacial European whitefish: rapid adaptive radiations into the littoral, pelagic, and profundal lake habitats, Ecology and evolution Res. 2013. Vol. 3. No 15. P. 4970–4986.
Pravdin I. F. Fish study guide. M.: Pisch. prom-st', 1966. 376 p.
Reshetnikov Yu. S. Kashulin N. A. Lukin A. A. The smallest whitefish in Europe, Pervyy Kongress ihtiologov Rossii: Tez. dokl. Astrahan'; M.: VNIRO, 1997. P. 50.
Reshetnikov Yu. S. Popova O. A. Kashulin N. A. Assessment of the well-being of the fish part of the aquatic community based on the results of fish morphological analysis, Uspehi sovremennoy biologii. 1999. No. 2. C. 165–177.
Reshetnikov Yu. S. Ecology and systematics of Coregoninae. M.: Nauka, 1980. 301 p.
Robinson B.W., Parsons K. J. Changing times, spaces, and faces: tests and implications of adaptive morphological plasticity in the fishes of northern postglacial lakes, Can. J. Fish. Aquat. Sci. 2002. Vol. 59. P. 1819–1833.
Schluter D. The ecology of adaptive radiation. Oxsford: Oxford University Press, 2000. 288 p.
Schluter D., Rambaut A., Clarke B. C. et al. Ecological speciation in postglacial fishes, Philosophical Transactions of the Royal Society B: Biological Sciences. 1996. Vol. 351. P. 807–814.
Sharov A. N. The structure of phytoplankton in the Far North reservoirs under the conditions of technogenic pollution: Dip. … kand. biol. nauk. Apatity, 2000. 23 p.
Sidorov G. P. Reshetnikov Yu. S. Salmonid fishes of the reservoirs of the European North-East. M.: T-vo nauch. izd. KMK, 2014. 346 p.
Siwertsson A., Knudsen R., Amundsen P, A. Temporal stability in gill raker numbers of subarctic European whitefish populations, Adv. Limnol. 2008. Vol. 63. P. 229–240.
Siwertsson A., Knudsen R., Kahilainen K. K. et al. Sympatric divercification as influenced by ecological opportunity and historical contingency in a young species lineage of whitefish, Evol. Ecol. Res. 2010. Vol. 12. P. 929–947.
Skúlason S., Snorrason S., Jónsson B. Sympatric morphs, populations and speciation in freshwater fish with emphasis on arctic charr, Magurran AE, May RM (eds.). Evolution of biological diversity. Oxford: Oxford University Press, 1999. P. 70–92.
Yakovlev V. A. Hydrobiological studies of the internal waters of the Kola North. Apatity: Izd-vo KNC RAN, 1991. 53 s
Ylikörkkö J., Zueva M., Kashulin N. et al. Environmental Challenges in the Joint Border Area. Reports 41. Centre for Economic Development, Transport and the Environment for Lapland. Kokkola: Juvenes Print, 2015. 165 p.
Zubova E. M. Linear growth of the European whitefish Coregonus lavaretus (L.) in anthropogenically modified water bodies of the European subarctic (on the example of Murmansk region): Dip. … kand. biol. nauk. Perm': PGNIU, 2015. 28 p.





 © 2011 - 2026
© 2011 - 2026